Host Immune Response to Chlamydia Infection
Host Immune Response to Chlamydia Infection
1. Introduction
From the immunological point of view, the infections produced by intracellular bacteria have several particular features:
1. protection against intracellular bacteria is mediated by T cells, which are interacting, not directly with the pathogen, but with the infected cell surface; antibodies exhibit a minor effect on the immune protection against intracellular infections;
2. infections by intracellular bacteria is accompanied by delayed hypersensitivity reactions that occur after the local administration of soluble antigens, mediated by T cells, whose effectors are macrophages;
3. tissular reactions to anti- intracellular bacteria are granulomatous in nature, both the protective responses and pathology being caused by them. Breaking granuloma favors pathogen dissemination and extension of lesions;
4. intracellular bacteria are expressing little or no toxic effects to the host cell, and pathology results from the activation of the immune response, mediated primarily by T lymphocytes. In contrast, extracellular bacteria secrete extracellular toxins, some of them being extremely potent and producing direct tissue damage;
5. intracellular bacteria are well adapted for coexisting with the host cell for long periods, by maintaining a balance between the persistent infection and the protective immunity mechanisms, resulting in a long incubation period and the development of a chronic infectious process. The infection per se is distinct from the pathological process. In contrast, extracellular bacteria cause acute infections, which are triggered soon after infection and ending after the immune response reaches an optimal intensity.
Some pathogenic bacteria such M. tuberculosis, M. bovis, M. leprae, S. enterica, Brucella sp., L. monocytogenes, Francisella tularensis, are facultatively intracellular, passing through a intracellular phase during their infectious cycle, without being strictly dependent on the cellular environment. They could infect primarily monocytes, but also other cells. In contrast, strictly intracellular parasitic bacteria (Chlamydia, Rickettsia) do not survive in the extracellular environment of the host. They infect endothelial and epithelial cells, and monocytes.
2. Life cycle of clamydiales
Chlamydiae are Gram-negative intracellular bacteria found in a wide variety of hosts. Chlamydia species infectious to humans are: Chlamydophila psittaci, Chlamydia trachomatis (biovar lymphogranuloma venereum - LGV and trachoma) and Chlamydia pneumoniae. Differentiation is based on their antigenic composition, susceptibility to sulfonamides and pathogenesis. Chlamydia pecorum is infectious to animals, but not to humans. Chlamydophila psittaci is parasitic on birds and mammals, and much more rarely to cold-blood vertebrates and humans. Chlamydia trachomatis is parasitic for mice and humans. In mammals and birds, both species produce eye conjunctiva, urogenital, respiratory and digestive tract infections and possibly at other sites (Barnes, 1990).
Chlamydia species have similar morphology and a common development cycle. By adapting to the intracellular compartment, chlamydiae lost a lot of the energy producing metabolic ways and the ability to produce ATP, but preserved some major metabolic activities, such are: glycolysis, respiration, pentoses biosynthesis. Development of intracellular chlamydiae depends on ATP and other high energy compounds derived from the host cell. Chlamydiae possess the enzymatic systems required for DNA, RNA and proteins synthesis, but they use many of the host cell precursors (nucleotides, amino acids, nutrients, vitamins, various cofactors) (Madigan et al., 1997).
In terms of structure, there were identified two chlamydia types: the infectious, nonreplicating elementary body (EB) and the noninfectious, but actively replicating reticulate body (RB), which are alternating in the development of the infectious cycle. The two morphologically distinct forms - the EBs and RBs - are representing adaptations to the extracellular environment and respectively to the intracellular one. The infectious particle, stable in the extracellular environment is a small cell, called the elementary body. They enter the host cell, multiply and disseminate in the extracellular environment. To complete these steps, chlamydiae undergo a developmental cycle that includes forms with alternating morphology and function.
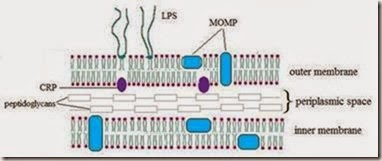
Chlamydia cell wall is similar to the Gram-negative bacteria, with increased lipid content, but does not contain peptidoglycan (Hatch, 1996) (Fig. 1). Pentapeptidic similar components may be present, because their growth and division is altered in the presence of β-lactam antibiotics.
Fig. 1. Structure of the Chlamydia cell wall (membrane Momp major out membrane protein, CRP, cysteine-rich protein)
The outer membrane of Chlamydia pneumoniae is composed of lipopolysaccharides (LPS) and heat-shock proteins (HSP) that are genus specific (Matsumoto et al., 1991, Shirai et al., 2000). The bacterial family of Chlamydiaceae comprises a group of obligate intracellular pathogens which harbour a highly truncated LPS, being composed of KDO (2-keto-3-deoxyoctonate) units only. In all chlamydial species, the linear trisaccharide a-KDO-(2 - 8)-a-KDO-(2 - 4)-a- Kdo has been found, thereby constituting a family specific antigen. Antibodies raised against these neoglycoconjugates displayed a wide range of specificities and affinities.
Several major outer membrane proteins (MOMP) identified are detectable by monoclonal antibodies and are species specific (Matsumoto et al., 1991, Shirai et al., 2000). Under these persistence-inducing conditions, the chlamydial Hsp60 appears to be highly upregulated. Chlamydial Hsp60 has proinflammatory effects by directly activating mononuclear leukocytes, which are mediating the inflammatory response. Chlamydial persistence is characterized by morphologically aberrant RBs that do not undergo cytokinesis, considered a third chlamydial type, called the noninfectious, nonreplicating persistent body (PB). Persistent Chlamydia, however, re-enter the productive life cycle upon removal of the stress factor.
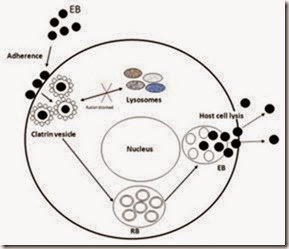
The infectious chlamydial form is the EB, which is adhering to the target cell membrane and initiates penetration. Elementary body is small, dense, with a rigid cell wall, given by the multiple SS bonds and to two other major outer membrane proteins (CRPs): a wall protein of 60 kDa and the outer membrane lipoprotein of 12 kDa. EB is resistant to environmental factors favouring survival in the extracellular environment after cell lysis and switching from one cell or one host to another. EB is not dividing, is metabolically inert, but has high affinity for epithelial cells. Its role is to spread the infection from one cell to another. Its membrane contains heparan-sulphate (proteoglycan), a ligand, through which they are attached to the microvilli and subsequently embedded in clatrin vesicles by receptors mediated endocytosis. Chlamydiae remain in the intracellular vacuole with non acid pH and avoid the fusion of the phagocytic vesicle with the lysosomes by unknown mechanisms. Inside the large vacuole, chlamydiae are redistributed from the cell periphery to the nucleus periphery by a mechanism dependent on actin F. The EB undergoes morphological changes and reorganization in the RB which is larger, metabolically active, and divides repeatedly by binary fission and becomes visible as a microcolony known as chlamydial inclusion. RB contains 4 times more RNA than DNA and RNA, while the EB contains equal proportions of RNA and DNA.
The EB - RB conversion requires the alteration of the outer membrane structure by the cleavage of SS bonds. Reduction of SS bonds leads to increased membrane fluidity, increased permeability, favours the nutrients transport from the host cell and the intiation of metabolic activity. After a period of growth and division, EB is reorganized; it condenses and forms numerous EBs. Development cycle is complete, with the host cell lysis and subsequent release of EB which are initiating a new infection cycle (fig. 2). The complete development cycles, when studied in cell cultures, is taking 47-72 hours and vary depending on the strain, the host cell and the environmental conditions (Campbell & Kuo, 2004).
The pathological processes due to chlamydial infection appear to be mediated by the immune reactivity. The host immune response stimulated by repeated episodes of infection increases the pathological lesions. Reinfection or persistence state causes pathological
changes. Persistence of infection has been shown in different cell cultures being associated with PBs, which remain viable but not replicating, suggesting an innate ability of chlamydiae to persist intracellularly, and the mechanism by which they induce a pathological process. The factors favouring the persistence are unknown.
Fig. 2. Life cycle to Chlamydia pneumoniae
Chlamydiae, and other intracellular pathogens, are circumventing the normal mechanisms of host cell defences. Endocitated chlamydiae are sequestered in the host cell membrane derived phagosome. The key event that allows the survival of the infectious agent in the host cell is the inhibition of phagosome–lisosome fusion. Persistently infected cultures are less sensitive to rifampicin and clortetracycline, which suggests that chlamydiae DNA transcription and translation continues in the persistent forms.
In vitro, chlamydia can invade host cells deficient in nutrients and after the entry they adopt a dormant, non-infectious, but viable state. The persistence of the non-replicative stage is reversible. By adding for example, L-isoleucine, an essential aminoacid for chlamydiae and host cells is sufficient to enable development of chlamydiae. It is believed that the persistence of chlamydiae is the result of competition between host and parasitic cells for L- isoleucine. The treatment with cicloheximide, an inhibitor of host cell protein synthesis - stops the competition between the host cell and chlamydiae for L-isoleucine that remains available for bacterial protein synthesis. Chlamydiae induce synthesis of interferon that has been shown to inhibit pathogen growth in cell culture.
3. Immunological aspects of acute and chronic Chlamydia infection
The protective immunity for Chlamydia pneumoniae and Chlamydia trachomatis infections is quite similar. Innate immunity is of key importance in primary recognition of invading pathogens. Infected epithelial cells respond in similar, but not identical ways to different invading pathogens and the pathogens are capable of modifying the host cell response (Severin & Ossevarde, 2006).
Cell-mediated immunity and especially participation of type 1 Th cells are crucial for eradication or limitation of the infection at a culture negative stage. Primary infection induces development of antigen-specific immunity. Chlamydia pneumoniae is generally transmitted from person to person via the respiratory route (Kuo et al., 1995, Grayston, 2002), where mechanical barriers and innate immune mechanisms comprise the first defense systems of the host. Epithelial cells lining the trachea and nasopharynx are the first cellular barriers against inhaled pathogens. Infection of airway epithelial cells can trigger a preliminary cascade of pro- and antiinflammatory immune reactions (secretion of IL-8, and expression of the epithelial adhesion molecule-1) that initiate drifting of polymorphonuclear neutrophils (PMN) and acute inflammation. Trachoma is the leading infectious cause of blindness. Eighty-five million children have active (inflammatory) trachoma, and about 7 million people, mainly adults, are blind from late scarring sequelae (Thylefors et al., 1995).
In trachoma, ocular Chlamydia trachomatis infection causes inflammatory changes in the conjunctiva, and repeated infections sometimes lead to fibrosis and scarring of the sub tarsal conjunctiva. The reasons for the heterogeneity in susceptibility to chlamydial infection and disease progression following a rather uniform bacterial exposure remain incompletely understood, but however, it has been postulated that the early secretion of pro- inflammatory cytokines and chemokines by epithelial cells following Chlamydia trachomatis infection may initiate and sustain a chronic inflammatory process associated with pathology (Stephens, 2003).
Natividad et al. (2009) tried to investigate how genetic variation detected in IL8 and CSF2 (colony stimulating factor) could affect risk of trachoma: IL8, CSF2 and MMP9 (matrix- metallo proteinase) are co-expressed in the Chlamydia trachomatis infected conjunctiva and these gene products could interact at the site of infection to augment and sustain inflammatory processes. MMP9 enhance the activity of IL8, whereas activation of neutrophils by IL-8 may trigger the release of pro-MMP9, creating a potential for a positive feedback loop. Similarly, CSF2 release by the infected epithelial cells may mediate the influx and activation of inflammatory cells at the site of infection. Secreted CSF2 may trigger MMP9 production by monocytes, which could act to enhance and sustain the pro- inflammatory cascade initiated by CSF2.
The onset of acquired antigen-specific immune responses depends on the speed of microbial dissemination from the initial site to local lymph nodes (Lipscomb et al., 1995). A preliminary pulmonary infection occurs in the alveoli, where the invading Chlamydia undergoes phagocytosis by dendritic cells (Prebeck et al., 2001) or alveolar macrophages (Nakajo et al., 1990; Redecke et al., 1998). The destruction of intracellular bacteria is dependent on the macrophage activation and mediated by reactive oxygen and nitrogen intermediates.
Chlamydia can protect against the microbicidal systems of the activated macrophages by inhibiting the phagolysosomal fusion and replicating in a special nonacidic chlamydial inclusion (Mihaescu et al., 2009). The migration of some infected macrophages and dendritic cells to regional lymph nodes contributes significantly to the initiation of the antigen-specific immune response (Lipcomb et al., 1995) The acquired immune response, both humoral and cell-mediated in Chlamydia pneumoniae and Chlamydia trachomatis infection is detectable from 1 to 2 weeks after the primary infection. Monocytes/macrophages and dendritic cells participate in the development of antigen-specific cell-mediated immunity by: (1) acting as antigen presenting cells (APC) and by (2) secretion of pro- IL-6, IL-1, IL-12) and antiinflammatory (IL-10, IL-13, cytokines, which activate other immune cells. In particular, the balance of IL-12 and IL-10 is crucial in regulating the development and functional characteristics of T cell responses (Surcel et al., 2005).
Chlamydial peptides processed by professional APC are further presented by MHC class II molecule to T CD4+ cells, although some chlamydial peptides are available also for MHC class I presentation. Activation of type 1 Th cell responses by IL-12 and IL-18 is considered the most important cytokine controlling chlamydiae infections, by regulating the cytotoxic T cells and by direct induction of nitric oxide synthase and the nitric oxide production in the macrophages as well as tryptophan depletion that inhibit chlamydial growth (Surcel et al., 2005). Activation of a type 2 cytokine response is associated with increased susceptibility to Chlamydia trachomatis and Chlamydia pneumoniae infection in experimental animal models.
Protective impact of T cells involves promotion of type 1 responses by activating other inflammatory cells, monocytes and macrophages, and cytotoxic T cells and B cells via cytokine secretion. CD4+ T cell activation generally dominates over CD8+ in in vitro and in vivo studies (Halme et al., 2000) especially in later stages of infection.
In primary infection in healthy people, circulating lymphocytes respond equally to Chlamydia pneumoniae and Chlamydia trachomatis antigens, suggesting that conservative chlamydial structures dominate over the species-specific ones as targets for the cell- mediated responses (Halme et al., 1997). The immune escape of chlamydiae within the host includes mechanisms for evasion of T cell recognition by interfering with the expression of MHC molecules. Chlamydia pneumoniae can suppress MHC class II expression on human monocytes (Airenne et al., 2002) or MHC class I expression on a monocytic cell line (Caspar- Baughil et al., 2000). In this process, Chlamydia pneumoniae secretes a proteolytically active molecule (Fan et al., 2002) a homologue of chlamydial protease-like activity factor of Chlamydia trachomatis, which can degrade constitutive transcription factors RFX5 and USF-1 molecules needed for antigen transcription during microbial infection (Zhong et al., 2001) . IL-10 by infected macrophages seems to inhibit the expression of MHC molecules (Caspar- Banguil et al., 2000).
Concerning the antibodies responses in chlamydiae infection, IgM response appears 2–3 weeks after the first symptoms of the illness and IgG response after 6–8 weeks. IgA antibodies are only occasionally found during the primary infection but appear frequently in the reinfection. The main antigens triggering an antibody mediated response are LPS and HSP-60. The protective value of these antibodies is still to be established.
Most of the times, especially in the infectious processes, immune responses are beneficial, having a role in eliminating pathogens. Sometimes, however, after the contact with some antigens (in particular molecular ones), activation of immune function is damaging and unfavorable for the host, causing two types of clinical manifestations: hypersensitivity and autoimmune diseases. Decreased immune system activity, generates a particular type of clinical manifestations generically known as immunodeficiencies. They can be congenital (primary) or acquired (secondary).
The state of hypersensitivity (HS) is a consequence of the fact that the primary immunization after the contact with the antigen and generation of immune effectors (antibodies and effector lymphocytes) do not give always a positive state, i.e. the resistance of the organism to an infection. Primary contact with the antigen creates sometimes a state of alert to the respective antigen, and at the second contact with the respective antigen, the human body responds by pathological hypersensitivity states, characterized by too high intensity or inappropriate immune response, which are the origin of tissue damage. The equivalent term commonly used for hypersensitivity is the allergy (allos, ergon, greek = other energy).
Autoimmunity is essentially an immune response against self antigens, which initiates the autoimmune conflict. One of the axioms of Immunology, that the host normal immune reactivity, does not produce antibodies against the body's own constituents, is frequently violated because the immune system turns against its own tissue components, disrupting the state of tolerance. Especially in the elderly, there are synthesized autoantibodies or autoreactive lymphocytes could occur (Lazar et al., 2005; Mihaescu et al., 2009).
By extending its effects, the autoimmune conflict can lead to autoimmune diseases characterized by the proliferative expansion of lymphocytes reactive against self components and generated effectors (autoreactive cells and antibodies) (Mihaescu and Chifiriuc, 2009). There are diseases with autoimmune phenomena well expressed, but their primary etiology is unknown. Among the variate range of acute infections caused by Chlamydia pneumoniae (pharyngitis, bronchitis, pneoumonia), late complications (asthma, allergic rhinitis, heart disease, multiple sclerosis, Alzheimer's) could occur (Friedman et al., 2004).
Chlamydia could induce a local T cell immunosuppression and inflammatory response revealing a possible host-pathogen scenario that would support both persistence and inflammation. For example, Chlamydia pneumoniae could inhibit activated, but not nonactivated human T cell proliferation in a pathogen specific maner, heat sensitive, and multiplicity of infection dependent. The Chlamydia pneumoniae antiproliferative effect is linked to T cell death associated with caspase 1, 8, 9, and IL-1β production, indicating that both apoptotic and proapoptotic cellular death pathways is activated after pathogen-T cell interactions (Olivares-Zavaleta et al., 2011).
Although T-cell activation during acute Chlamydia pneumoniae infections has been described, little is known about the frequency or the role of the Chlamydia pneumoniae-specific memory T cells that reside in the human body after the resolution of the infection. The analysis of Chlamydia pneumoniae-induced T-cell responses in peripheral blood mononuclear cells showed that following short-term stimulation with Chlamydia pneumoniae, both gamma interferon (IFN-γ)- and interleukin-2 (IL-2)-producing CD4 (+) T-cell responses could be detected in some PBMC culture from healthy individuals. Chlamydia pneumoniae-activated CD4(+) T cells expressed CD154, a marker for T-cell receptor-dependent activation, and displayed a phenotype of central memory T cells showing dominant IL-2 production but also IFN-gamma production.
Interestingly, individuals with both IFNγ and IL-2-producing responses showed significantly decreased immunoglobulin G reactivity toward Chlamydia pneumoniae RpoA and DnaK, antigens known to be strongly upregulated during chlamydial persistence, compared to IgG reactivity of seropositive individuals with no T-cell response or CD4(+) T- cell responses involving the production of a single cytokine (IFN-γ or IL-2). Among seropositive individuals, the presence or the absence of dual IFN-γ and IL-2-producing T- cell responses was associated with distinct patterns of antibody responses toward persistence-associated Chlamydia pneumoniae antigens.
4. Chlamydia pneumoniae infection and chronic diseases with immunopathological background
There are many seroepidemiological, pathological, animal, immunological and antibiotic treatment studies demonstrating the role of chlamydial infection in atherosclerosis. EBs of Chlamydia pneumoniae is internalized by endocytosis in the alveolar macrophages, and differentiates in RBs, which are multiplying. Circulating monocytes are infected and the appearance of chlamydial inclusions in the vascular endothelium modulates their adhesion. The adhered macrophages migrate later in the intimate of blood vessels, where they cause infection of other cell types, including arterial macrophages, which will start to accumulate LDL (low density lipoproteins), getting a "foamy" aspect because of the stored cholesterol. Under nonatherogenic conditions, LDL uptake causes the transcriptional downregulation of its receptor. Scavenger receptors, however, may bypass this control by allowing the endocytosis of modified LDL. Chlamydia pneumoniae heat shock protein–60 (cHsp60) was found to induce cellular oxidation of LDL cholesterol, while Chlamydia pneumoniae LPS as the antigen that could enhance LDL cholesterol uptake and downregulate cholesterol efflux in monocytes and macrophages (Kalayoglu et al., 2002; Higgins, 2003).
Smooth muscle fibers will also be infected by the EBs and will proliferate, and infected endothelial cells will secrete a number of cytokines, which will ultimately lead to the destabilization of the ateromatous plaque and thrombus formation, with an increased risk of myocardial infarction.
Currently, serological methods could fail in detecting chronic or persistent Chlamydia pneumoniae infection and other blood markers such as Chlamydia pneumoniae immune complexes and circulating leukocytes, e.g. macrophages with detectable Chlamydia pneumoniae DNA and mRNA as markers of underlying infection are needed (Ngeh et al., 2002; Ngeh & Gupta, 2002). The association of some inflammatory markers such as C- reactive protein (CRP) with Chlamydia pneumoniae serology has indicated the existence of some correlations between “infectious” and “inflammatory” burdens and atherosclerosis (Leinonen & Saikku, 2002). Cytokines released by these immune cells, such as IL-6, induce acute phase response proteins, and elevated CRP levels, (a marker for atherosclerosis) are an indication of chronic inflammation. The inflammatory nature of the early atheroma causes modifications to LDL, which allow the molecule to bypass the usual regulatory mechanisms for entry into cells, thus facilitating foamy cell formation. Furthermore, the effect of inflammation on endothelial cells allows for greater arterial permeability to LDL and increased adhesion molecule expression and diapedesis of leukocytes.
The presence of Chlamydia pneumoniae was evidenced by different methods including immunocytochemistry, PCR, in situ hybridization and electron microscopy not only in coronary arteries, but also in cerebral, carotid, internal mammary, pulmonary, aorta, renal, iliac, femoral, and popliteal arteries, and occluded bypass grafts, obtained from postmortem and surgical atheromatous tissues (Leinonen & Saikku, 2002; Ngeh et al., 2002, Ngeh & Gupta, 2002, Rassu et al., 2001). However, it must be taken into account that Chlamydia pneumoniae can also be detected in about 10% of noncardiovascular and granulomatous tissues, showing a nonspecific, ubiquitous distribution in the human body (Ngeh et al., 2002, Ngeh & Gupta, 2002).
Animal models have shown that Chlamydia pneumoniae infection could initiate and accelerate atherosclerotic process. Repeated Chlamydia pneumoniae infection in genetically modified mouse such as ApoE-deficient mouse known to develop atherosclerosis in the absence of a fatty diet in has been shown to accelerate atherosclerotic lesion progression by specifically increasing the T lymphocyte influx in the atherosclerotic plaques and accelerating the formation of more advanced atherosclerotic lesions (Ezzahiri et al., 2002).
The exact pathogenesis of multiple sclerosis (MS), the most common demyelinating disease of the central nervous system (CNS) (Noseworthy, 1999; Nosewrothy et al., 2002) remains unknown, but current hypothesis are reffering to an autoimmune background that may be influenced by an infectious process. Chlamydia pneumoniae has been found to be associated with the rapidly progressive multiple sclerosis (Sriram et al., 1998), or relapsing multiple sclerosis, markedly improved after targeted antimicrobial therapy (Sriram et al., 1999)
The association between Chlamydia pneumoniae and multiple sclerosis was demonstrated in experimental pathogenesis, showing that after intraperitoneal inoculation of mice with Chlamydia pneumoniae, following immunization with neural antigens, an increased severity of experimental allergic encephalitis (EAE), an animal model of multiple sclerosis was observed. An attenuation of EAE following therapy with the antimicrobial agent fluorphenicol (Du et al., 2002) was observed. Persistence of Chlamydia pneumoniae in the CNS is likely to provide an environment that can lead to the activation of autoreactive T cells and contribute to the pathogenesis of a chronic disease such as multiple sclerosis (Stratton & Sriram, 2004).
Alzheimer’s disease (AD) is a progressive neurodegenerative condition that accounts for the most common and severe form of dementia in the elderly The pathology observed in the brain includes neuritic senile plaques, neurofibrillary tangles, neuropil threads and deposits of cerebrovascular amyloid (Balin et al., 2005).
Balin et al. (1998) demonstrated by polymerase chain reaction (PCR) that the DNA of Chlamydia pneumoniae was present in 90% of postmortem brain samples examined from sporadic AD.). Immunohistochemistry revealed the presence of Chlamydia pneumoniae antigens in perivascular macrophages, microglia, and astroglial cells in areas of the temporal cortices, hippocampus, parietal cortex, and prefrontal cortex in AD patients, but not in control samples. Electron microscopy revealed chlamydial inclusions that contained elementary (EB) and reticulate (RB) bodies. Microglia responds to insult with the production of proinflammatory cytokines, and the generation of reactive oxygen species and other products (Simpson et al., 1998). Monocytes acutely or chronically in vitro infected with Chlamydia pneumoniae appeared to increase the expression of amyloid precursor protein as well as the increased breakdown of the precursor into fragments that contained 1–40 immunoreactive epitopes, generating the initial focal points for amyloid deposition (Balin et al., 2004).
The way by which Chlamydia pneumoniae croos the blood–brain barrier to reach the central nervous system is probably represented by the circulating monocytes infected with Chlamydia pneumoniae (Boman et al., 1998; Airenne et al., 1999). The transmigration is facilitated by the increased surface expression of the surface adhesins on the endothelial cells and the integrins on the monocytes. Chlamydia pneumoniae may reach into the central nervous system through the the olfactory neuroepithelium of the nasal olfactory system, in direct contact with the infected epithelial cells.
Reactive arthritis is a sterile immune-mediated pathogenesis process of the joint that follows bacterial infection of either the gastrointestinal or urogenital system (Whittum-Hudson et al., 2004). Chronic reactive arthritis associated with chlamydial infection is manifested with activation of TH1/TH2 CD4+ cells and macrophages at sites of inflammation (Carter & Dutton, 1996). In addition, at sites of chlamydial infections, proinflammatory cytokines IL-6, and TH1-associated cytokines and IL-12 have been identified (Mosmann & Coffman, 1989).
It is not clear whether chlamydial infection elicits an inflammatory response because of upregulated cytokine production in infected or neighbouring cells, or if the immune response to infected cells drives the inflammatory response via influx of cytokine-producing lymphocytes and macrophages. Nonetheless, synovial materials have been studied for the panel of cytokines present in Chlamydia trachomatis- and Chlamydia pneumoniae–induced inflammatory arthritis. After development of inflammation, Th1/Th2 CD4+ cells, as well as CD8+ cells and macrophages, have been detected in synovial fluid (Simon et al., 1993). Many studies have indicated that, in patient materials from individuals with early disease, proinflammatory cytokines such as IL-12 and are prominent (Kotake et al., 1999; Simon et al., 1993). In Chlamydia trachomatis–infected joint tissues from chronic reactive arthritis patients, IL-10, IL-8, IL-15 and MCP-1t (Gérard et al., 2002) have been identified. In the same study, it was shown that in synovial tissue samples from arthritis patients chronically infected at that site with Chlamydia pneumoniae, essentially only mRNA encoding IL-8, and RANTES were present.
Acute Chlamydia pneumoniae infection can cause acute bronchitis and pneumonia, and lower respiratory tract illnesses can develop into asthma and chronic bronchitis. Chronic Chlamydia pneumoniae infection has also been associated with a wide variety of chronic upper-airway illnesses, as well as with acute and chronic lower-airway conditions including acute bronchitis, asthma and COPD.
Chlamydia pneumoniae infection could have three distinguishable causal effects: acute asthma exacerbations (Allegra et al., 1994, Clementsen et al., 2002); promoting asthma severity (von Hertzen, 2002); or initiate asthma (Hahn et al., 2005).
A significant association between asthma and Chlamydia pneumoniae-specific IgA (Hahn et al., 2000, Genkay et al., 2001, Falk et al., 2002) and also with chlamydial heat shock protein–
60 (Hsp60) antibodies (Roblin et al., 2000, Huttinen et al., 2001) has been reported by different authors.
It is already established that the inflammatory processes of chlamydial pathogenesis are elicited by infected host cells and are necessary and sufficient to account for chronic and intense inflammation and the promotion of cellular proliferation, tissue remodelling and scarring, the ultimate cause of disease sequelae (Stephens, 2003).
One mechanism by which infection can initiate autoimmune disease is molecular mimicry, the phenomenon of protein products from dissimilar genes sharing similar structures that elicit an immune response to both self and microbial proteins. The strongest cases for molecular mimicry seem to have been made for chlamydial heat shock proteins 60, the DNA primase of Chlamydia trachomatis, and chlamydial OmcB proteins (Bachmaier & Penninger, 2005). So, the HSPs are expressed by cells within atherosclerotic plaques, and anti-HSP antibodies serum titres have been reported to be positively related to future risk of coronary heart disease. On the other hand, purified anti-HSP antibodies recognise and mediate the lysis of stressed human endothelial cells and macrophages in vitro and future immunisation with HSP exacerbates atherosclerosis in experimental animal models. Taking into account that some human vaccines, such as BCG, contain HSPs, hence although vaccination programmes are vital for maintaining ‘herd’ immunity and the prevention of serious infectious disease, they may leave a legacy of increased susceptibility to atherosclerosis (Lamb et al., 2003).
To investigate the conditions under which autoimmune responses can be generated against self hsp60, Yi et al., (1997) demonstrated that autoimmune responses characterized by strong T-cell proliferation and high titers of antibody to self hsp60 are induced only by concurrent immunization with mouse and chlamydial hsp60. They observed that switches in cytokine production patterns may mediate the pathogenesis of hsp60-associated Chlamydia trachomatis immunopathology. Immunization with mouse hsp60 alone induced lymphocytes that secreted high levels of interleukin-10 (IL-10) but did not proliferate in response to in vitro stimulation with mouse hsp60. On the other hand, co-immunization with mouse and chlamydial hsp60s induced lymphocytes that proliferated strongly in response to mouse hsp60, secreted 6-fold less IL-10, and exhibited a 12-fold increase in the ratio of IFNγ /IL-10 production (Yi et al., 1997). The development of infertility is reported due to enhanced immune responses to Chlamydia trachomatis (Debattista et al., 2003), and cHSP60 and cHSP10 antibodies seem to perform well in predicting tubal factor infertility (Spandorfer et al., 1999; LaVerda et al., 2000; den Hartog et al., 2005; Dadamessi et al., 2005; Linhares & Witkin, 2010). During Chlamydia infection a crucial for controlling the duration of infection and subsequent tubal pathology have Th1/Th2 responses: Th1 cells produce IFN-γ that promotes the destruction of Chlamydia (Beatty et al., 1993), but can also promote inflammatory damage and fibrosis (Rottenberg et al., 2002) whereas Th2 cells produce IL-4, IL-5, and IL-13 believed to be critical for defense against extracellular pathogens. The production of TNF-α and IL-10 was examined because their levels have been reported to be high in cervical secretions of Chlamydia trachomatis infected infertile women (Reddy et al., 2004). In order to elucidate the actual role in the cause of infertility, Srivastava et al., (2008) studied the specific cytokine responses of mononuclear cells from the infectious site to cHSP60 and cHSP10. Exposure to chlamydial heat shock proteins (cHSP60 and cHSP10) could significantly affect mucosal immune function by increasing the release of IFN-γ, IL-10 and TNF-alpha by cervical mononuclear cells, much more in infertile group as compared to fertile group.
5. Conclusion
Chlamydia infection has excited considerable attention in the last time research, not only as a genital or respiratory pathogen but because of its association with a number of acute and chronic diseases. The true significance of Chlamydia infection in the development of chronic manifestations still remains puzzling. The factors that drive immune responses to pathogenic species, the role of host genetic background, HLA molecules and cytokine gene polymorphism, environmental and epidemiological factors, mixed infections, and species or dose of the infecting agent probably all interact in a final balance of the immune defense mechanisms. Chlamydial infections in vivo typically result in chronic inflammation characterized cellularly by the presence of activated monocytes and macrophages and by the secretion of Th-1/Th-2 cytokines, which could lead to the dysregulation of the immune response resulting in autoimmune diseases. Understanding the mechanisms by which chlamydial infections, especially chronic and persistent ones interfere with the host immune defence system is necessary to develop better therapies to treat and possibly even prevent Chlamydia associated diseases. However, the early identification and treatment of genital chlamydial infection of women, of eye infection in children and of Chlamydia pneumoniae in chronic airways disease will be important to prevent the development of chronic sequelae, with immunological basis.
6. Acknowledgment
Acknowledgement: to the ANCS bilateral project Romania –Republic of Moldavia, no 423/04.06.2010 and National Project PN2 42119/2008 and PN2 42150/2008.
7. References
Airenne, S.; Kinnunen, A.; Leinonen, M.; Saikku, P. & Surcel, H.M. (2002). Secretion of IL-10 and IL-12 in Chlamydia pneumoniae infected human monocytes, presented at Chlamydial Infections Proceedings of the Tenth International Symposium on Human Chlamydial Infections, Antalya, Turkey.
Allegra‚ L.; Blasi‚ F.; Centanni‚ S.; Cosentini‚ R.; Denti‚ F.; Raccanelli‚ R.; Tarsia‚ P. & Valenti‚ V. (1994). Acute exacerbations of asthma in adults: Role of Chlamydia pneumoniae infection‚ Eur. Respir.J. Vol. 7, pp. 2165–2168.
Airenne, S.; Surcel, H.M.; Alakarppa, H.; Laitinen, K.; Paavonen, J.; Saikku, P. & Laurila, A.
(1999). Chlamydia pneumoniae infection in human monocytes, Infect. Immun. Vol. 67, pp. 1445–1449. [published erratum appears in Infect. Immun. (1999) 67:6716].
Bachmaier, K. & Penninger, J.M. (2005). Chlamydia and antigenic mimicry. Curr Top Microbiol Immunol. Vol. 296, pp.153-63.
Balin, B.J.; Hammond, C.J.; Little, C.S.; Macintyre, A. & Appelt, D.M. (2005). Chlamydia pneumoniae in the Pathogenesis of Alzheimer’s Disease, In: Chlamydia pneumoniae: Infection and disease. Friedman H., Yamamoto Y, Bendinelli M. pp.
211-226, Ed. Kluwer Academic Publishers, ISBN 0-306-48487-0, New York.
Balin, B.J.; Gerard, H.C.; Arking, E.J.; Appelt, D.M.; Branigan, P.J.; Abrams, J.T.; Whittum-Hudson, J.A. & Hudson, A.P. (1998). Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain, Med. Microbiol. Immunol. Vol. 187, pp. 23–42.
Barnes, R.C. (1990). Infections Caused by Chlamydia trachomatis, In Sexually Transmitted Diseases. Morse. SA, Moreland AA, Thompson SE, eds, J.B. Lippincott. Philadelphia Beatty, W.L.; Byrne, G.I. & Morrison, R.P. (1993). Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA, Vol. 90, pp. 3998-4002.
Boman, J.; Soderberg, S.; Forsberg, J.; Birgander, L.S.; Allard, A.; Persson, K.; Jidell, E.; Kumlin, U.; Juto, P.; Waldenstrom, A. & Wadell, G. (1998). High prevalence of Chlamydia pneumoniae DNA in peripheral blood mononuclear cells in patients with cardiovascular disease and in middle-aged blood donors, J. Infect. Dis. Vol. 178, pp. 274–277.
Campbell, L.A. & Kuo, C.C. (2004). Chlamydia pneumoniae — an infectious risk factor for atherosclerosis? Nature Reviews Microbiology, Vol. 2, pp. 23-32.
Carter, L.L. & Dutton, R. W. (1996). Type 1 and Type 2: A fundamental dichotomy for all T- cell subsets, Curr. Opin. Immunol., Vol. 8, pp. 336–342.
Caspar-Bauguil, S.; Puissant, B.; Nazzal, D.; Lefevre, J.C.; Thomsen, M.; Salvayre, R. & Benoist, H. (2000). Chlamydia pneumoniae induces interleukin-10 production that downregulates major histocompatibility complex class I expression, J. Infect. Dis. Vol. 182, No. 5, pp. 1394-1401.
Clementsen‚ P.; Permin‚ H. & Norn‚ S. (2002). Chlamydia pneumoniae infection and its role in asthma and chronic obstructive pulmonary disease‚ J. Invest. Allergol. Clin. Immunol. Vol. 12, pp. 73–79.
Dadamessi, I.; Eb, F. & Betsou. F. (2005). Combined detection of Chlamydia trachomatis specific-antibodies against the 10 and 60-kDa heat shock proteins as a diagnostic tool for tubal factor infertility: Results from a case-control study in Cameroon. FEMS Immunol Med Microbiol, Vol. 45, pp. 31-35.
Debattista, J.; Timms, P.; Allan, J. & Allan, J. (2003). Immunopathogenesis of chlamydia trachomatis infections in women. Fertil Steril. Vol. 79, No. 6, pp. 1273-1287.
den Hartog JE, Land JA, Stassen FR, Kessels AG, Bruggeman CA: Serological markers of persistent Chlamydia trachomatis infections in women with tubal factor subfertility.
Hum Reprod 2005, 20:986-990.Debattista, J.; Timms, P.; Allan, J. & Allan, J. (2003).
Immunopathogenesis of Chlamydia trachomatis infections in women. Fertil Steril.
Vol. 79, pp. 1273-1287.
Du, C.; Yi-Yao, S.; Rose, A. & Sriram, S. (2002). Chlamydia pneumoniae infection of the central nervous system worsens EAE, J. Exp. Med., Vol. 196, pp. 1639–1644.
Ezzahiri, R.; Nelissen-Vrancken, H.J.M.G.; Kurvers, H.A.J.M.; Stassen, F.R.M.; Vliegen, I.;
Grauls, G.E.L.M.; van Pul, M.M.L.; Kitslaar, P.J.E.H.M. & Bruggeman, C.A. (2002).
Chlamydophila pneumoniae (Chlamydia pneumoniae) accelerates the formation of complex atherosclerotic lesions in Apo E3-Leiden mice, Cardiovasc. Res. Vol. 56, pp.
269–276.
Falck‚ G.; Gnarpe, J.; Hansson‚ L.O.; Svärdsudd‚ K. & Gnarpe‚ H. (2002). Comparison of individuals with and without specific IgA antibodies to Chlamydia pneumoniae.
Respiratory morbidity and the metabolic syndrome‚ Chest, Vol. 122, pp. 1587–1593.
Fan, P.; Dong, F.; Huang, Y. & Zhong, G. (2002). Chlamydia pneumoniae secretion of a protease-like activity factor for degrading host cell transcription factors is required for major histocompatibility complex antigen expression, Infect. Immun. Vol. 70, No.
3, pp. 345-349.
Gencay‚ M.; Rüdiger‚ J.J.; Tamm‚ M.; Solér‚ M.; Perruchoud‚ A.P. & Roth‚ M. (2001).
Increased frequency of Chlamydia pneumoniae antibodies in patients with asthma‚ Am.J.Respir. Crit. Care Med. Vol. 163, pp. 1097–1100.
Gérard, H.C.; Wang, Z.; Whittum-Hudson, J.A.; El-Gabalawy, H.; Goldbach-Mansky, R.; Bardin, T.; Schumacher, H.R. & Hudson, A.P. (2002). Cytokine and chemokine mRNA produced in synovial tissue chronically infected with Chlamydia trachomatis and Chlamydia pneumoniae, J. Rheumatol. Vol. 29, pp. 1827–1835.
Grayston, J.T. (2002) Background and current knowledge of Chlamydia pneumoniae and atherosclerosis, J. Infect. Dis. Vol. 181, S3, pp. S402-S410.
Hahn‚ D.L.; Peeling‚ R.W.; Dillon‚ E.; McDonald‚ R. & Saikku‚ P. (2000). Serologic markers for Chlamydia pneumoniae in asthma‚ Ann. Allergy Asthma Immunol. Vol. 84, No. 2, 227–233.
Hahn D.L. (2005). Role of Chlamydia pneumonia as an Inducer of Asthma. In Friedman H., Yamamoto Y, Bendinelli M. ed. Infectious agents and pathogenesis. Kluwer Academic/Plenum Publishers, pp. 1-10.
Halme, S.; Latvala, J.; Karttunen, R.; Palatsi, I.; Saikku, P. & Surcel, H.M. (2000). Cell- mediated immune response during primary Chlamydia pneumoniae infection, Infect. Immun. Vol. 68, No. 12, pp. 7156-7158.
Halme, S.; Syrjälä, H.; Bloigu, A.; Saikku, P.; Leinonen, M.; Airaksinen,J. & Surcel, H.M. (1997). Lymphocyte responses to Chlamydia antigens in patients with coronary heart disease, Eur. Heart J. Vol. 18, No. 7, pp. 1095-1101.
Hatch, T.P. (1996). Disulfide Cross-Linked Envelope Proteins: the Functional Equivalent of Peptidoglycan in Chlamydia? J. Bacteriol., Vol. 178, No. 1, pp. 1-5.
Higgins, J.P. (2003). Chlamydia pneumoniae and coronary artery disease: The antibiotic trials, Mayo Clin. Proc. Vol. 78, No. 3, pp. 321–332.
Kalayoglu, M.V.; Libby, P. & Byrne, G.I. (2002). Chlamydia pneumoniae as an emerging risk factor in cardiovascular disease, JAMA Vol. 288, pp. 2724–2731.
Kotake, S.; Schumacher, H.R.; Arayssi, T.K.; Gérard, H.C.; Branigan, P.J.; Hudson, A.P.; Yarboro, C.H.; Klippel, J.H. & Wilder, R.L. (1999). IL-10, and IL-12 p40 gene expression in synovial tissues from patients with recent-onset Chlamydia-associated arthritis, Infect. Immun. Vol. 67, pp. 2682–2686.
Kuo, C.C.; Jackson, L.A.; Campbell, L.A. & Grayston, J.T. (1995). Chlamydia pneumoniae (TWAR), Clin. Microbiol. Rev. Vol. 8, No. 4, pp. 451-461.
LaVerda, D.; Albanese, L.N.; Ruther, P.E.; Morrison, S.G.; Morrison, R.P.; Ault, K.A. & Byrne, G.I. (2000). Seroreactivity to Chlamydia trachomatis Hsp10 correlates with severity of human genital tract disease. Infect Immun, Vol. 68, No. 1, pp. 303-309.
Lamb DJ, El-Sankary W, Ferns GA. 2003 Molecular mimicry in atherosclerosis: a role for heat shock proteins in immunisation. Atherosclerosis. Vol. 167, No. 2, pp. 177-85.
Lazar, V.; Balotescu, M.C.; Cernat, R.; Bulai, D. & Stewart-Tull, D. (2005). Imunobiologie, Ed.
Univ. din Bucuresti, Bucharest, 250 p. ISBN-973-73-7124-0
Leinonen, M. & Saikku, P. (2002). Evidence for infectious agents in cardiovascular disease and atherosclerosis, Lancet Infect. Dis. Vol. 2, No. 2, pp. 11–17.
Linhares, I.M. & Witkin, S.S. (2010). Immunopathogenic consequences of Chlamydia trachomatis 60 kDa heat shock protein expression in the female reproductive tract.
Cell Stress Chaperones. Vol. 15, No. 5, pp. 467-473.
Lipscomb, M.F.; Bice, D.E.; Lyons, C.R.; Schuyler, M.R. & Wilkes, D. (1995). The regulation of pulmonary immunity, Adv. Immunol. Vol. 59, pp. 369-455.
Madigan, M.; Martinko, J.; Parker, J. (2008). In Brock's Biology of Microorganisms. (12th Edition), ISBN 0132324601, New Jersey: Prentice Hall.
Matsumoto, A.; Bessho, H.; Uehira, K. & Suda, T. (1991). Morphological studies of the association of mitochondria with chlamydial inclusions and the fusion of chlamydial inclusions, J. Electron Microsc. Vol. 40, No. 5, pp. 356–363.
Mihăescu, G. & Chifiriuc, M.C. (2009). Organizarea sistemului imunitar la vertebrate, 2009, in vol. Imunogenetică şi Oncogenetică, Ed. Academia Român .
Mihăescu, G.; Chifiriuc, C.; Ditu, L.M. (2009) Imunobiologie, Ed. Univ. din Bucuresti, 572 p., 978-973-737-734-0. Mosmann, T. R. & Coffman, R. L., (1989). TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties, Annu. Rev. Immunol. Vol. 7, pp. 145–173.
Nakajo, M.N.; Roblin, P.M.; Hammerschlag, M.R.; Smith, P. & Nowakowski, M. (1990).
Chlamydicidal activity of human alveolar macrophages, Infect. Immun. Vol. 58, No.
11, pp. 3640-3644.
Natividad, A.; Hull, J.; Luoni, G.; Holland, M.; Rockett, K.; Joof, H.; Burton, M.; Mabey, D.; Kwiatkowski, D.& Bailey, R. (2009). Innate immunity in ocular Chlamydia trachomatis infection: contribution of IL8 and CSF2 gene variants to risk of trachomatous scarring in Gambians. MC Med Genet. Vol. 10, pp.138.
doi:10.1186/1471-2350-10-138
Ngeh.J.; Anand, V. & Gupta, S. (2002). Chlamydia pneumoniae and atherosclerosis-what we know and what we don’t, Clin. Microbiol. Infect. Vol. 8, No. 1, pp. 2–13.
Ngeh, J. & Gupta, S. (2002). Inflammation and infection in coronary artery disease, in: Cardiology:
Current Perspectives (G.Jackson, ed.), Martin Dunitz Ltd., London., 125–144.
Noseworthy, J.H. (1999). Progress in determining the causes and treatment of multiple sclerosis, Nature, Vol. 399, 6738 Suppl., pp. A40–A47.
Noseworthy, J.H.; Lucchinetti, C.; Rodriguez, M. & Weinshenker, B.G. (2000). Multiple
Sclerosis, New. Eng.J. Med., Vol. 343, No. 13, pp. 938–946.
Olivares-Zavaleta, N.; Carmody, A.; Messer, R.; Whitmire, W.M. & Caldwell, H.D. (2011).
Chlamydia pneumoniae inhibits activated human T lymphocyte proliferation by the induction of apoptotic and pyroptotic pathways. J Immunol. Vol. 186, No. 12, pp. 7120-7126.
Prebeck, S.; Kirschning, C.; Durr, S.; da Costa, C.; Donath, B.; Brand, K.; Redecke, V.; Wagner, H. & Miethke, T. (2001). Predominant role of toll-like receptor 2 versus 4 in Chlamydia pneumoniae–induced activation of dendritic cells, J. Immunol. Vol. 167, No. 6, 3316-3323.
Rassu, M.; Cazzavillan, S.; Scagnelli, M.; Peron, A.; Bevilacqua, P. A.; Facco, M.; Bertoloni, G.; Lauro, F. M.; Zambello, R. & Bonoldi, E. (2001). Demonstration of Chlamydia pneumoniae in atherosclerotic arteries from various vascular regions, Atherosclerosis, Vol. 158, No. 1, pp.73–79.
Reddy, B.S.; Rastogi, S.; Das, B.; Salhan, S.; Verma, S. & Mittal, A. (2004). Cytokine expression pattern in the genital tract of Chlamydia trachomatis positive infertile women – implication for T-cell responses. Clin Exp Immunol, Vol. 137, No. 3, pp. 552-558.
Redecke, V.; Dalhoff, K.; Bohnet, S.; Braun, J. & Maass, M. (1998). Interaction of Chlamydia pneumoniae and human alveolar macrophages: Infection and inflammatory response, Am. J. Resp. Cell. Mol. Biol. Vol. 19, No. 5, pp. 721-727.
Rottenberg, M.E.; Gigliotti-Rothfuchs, A. & Wigzell, H. (2002). The role of IFN-gamma in the outcome of chlamydial infection. Curr Opin Immunol, Vol. 14, pp. 444-451.
Roblin‚ P.M.; Witkin‚ S.S.; Weiss‚ S.M.; Gelling‚ M. & Hammerschlag‚ M.R. (2000). Immune response to Chlamydia pneumoniae in patients with asthma: Role of heat shock proteins (HSPs)‚ in: Proceedings: Fourth Meeting of the European Society for Chlamydia Research‚ Helsinki‚ Finland‚ Esculapio‚ Bologna‚ Italy‚ p. 209.
Severin, J.A. & Ossewaarde J.M. (2006) Innate immunity in defense against Chlamydia trachomatis infections. Drugs Today (Barc). Vo. 42, Suppl A:75-81.
Shirai, M.; Hirakawa, H.; Kimoto, M.; Tabuchi, M.; Kishi, F.; Ouchi, K.; Shiba, T.; Ishii, K.; Hattori, S.; Kuhara, S. & Nakazawa, T. (2000). Comparison of whole genome sequencesof Chlamydia pneumoniae J 138 from Japan and CWL 029 from USA, Nucleic Acid Res. Vol. 28, No. 12, pp. 2311–2314.
Simon, A.K.; Seipelt, E.; Wu, P.; Wenzel, B.; Braun, J. & Sieper, J. (1993). Analysis of cytokine profiles in synovial T cell clones from chlamydial reactive arthritis patients: Predominance of the Th1 subset, Clin. Exp. Immunol. Vol. 94, pp. 122–126.
Simpson, J.E.; Newcombe, J.; Cuzner, M.L. & Woodroofe, M.N. (1998). Expression of monocyte chemoattractant protein-1 and other beta-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions, J. Neuroimmunol.Vol. 84, pp. 238–249.
Spandorfer, S.D.; Neuer, A.; LaVerda, D.; Byrne, G.; Liu, H.C.; Rosenwaks, Z. & Witkin, S.S. (1999). Previously undetected Chlamydia trachomatis infection, immunity to heat shock proteins and tubal occlusion in women undergoing in-vitro fertilization. Hum Reprod, Vol. 14, No. 1, pp. 60-64.
Sriram, S.; Mitchell, W. & Stratton, C. (1998). Multiple sclerosis associated with Chlamydia pneumoniae infection of the CNS, Neurology, Vol. 50, No. 2, pp. 571–572.
Sriram, S.; Stratton, C.W.; Yao, S.; Tharp, A.; Ding, L.; Bannan, J.D. & Mitchell, W.M. (1999).
C. pneumoniae infection of the CNS in MS, Ann. Neurol. Vol. 46, No. 1, pp. 6–14.
Srivastava, P.; Jha, R.; Bas, S.; Salhan, S. & Mittal, A. (2008). In infertile women, cells from Chlamydia trachomatis infected sites release higher levels of interferon-gamma, interleukin-10 and tumor necrosis factor-alpha upon heat-shock-protein stimulation than fertile women. Reprod Biol Endocrinol. Vol. 6, pp. 20, doi:10.1186/1477-7827-6-20.
Stephens RS. (2003). The cellular paradigm of chlamydial pathogenesis. Trends Microbiol.
Vol. 11, No. 1, pp. 44-51.
Stratton, C.W. & Sriram, S. (2005). Chlamydia pneumoniae as a candidate pathogen in multiple sclerosis. Chlamydia pneumoniae: Infection and disease. In: Chlamydia pneumoniae:
Infection and disease. Friedman H., Yamamoto Y, Bendinelli M. pp. 199-210, Ed.
Kluwer Academic Publishers, ISBN 0-306-48487-0, New York.
Surcel H.M. (2005). Chlamydia pneumoniae Infection and Diseases: Immunity to Chlamydia Pneumoniae, In: Chlamydia pneumoniae: Infection and disease. Friedman H., Yamamoto Y, Bendinelli M. pp. 81-98, Ed. Kluwer Academic Publishers, ISBN 0- 306-48487-0, New York.
Thylefors, B.; Negrel, A.D.; Pararajasegaram, R. & Dadzie K.Y. (1995). Global data on blindness. Bull World Health Organ, Vol. 73, No. 1, pp. 115-121.
Yi, Y.; Yang, X. & Brunham, R.C. (1997). Autoimmunity to heat shock protein 60 and antigen- specific production of interleukin-10. Infect Immun. Vol. 65, No. 5, pp. 1669-74.
von Hertzen‚ L.‚ Vasankari‚ T.‚ Liippo‚ K.‚ Wahlström‚ E. & Puolakkainen‚ M. (2002). Chlamydia pneumoniae and severity of asthma‚ Scand. J. Infect. Dis. Vol. 34, No. 1, pp. 22–27.
Whittum-Hudson, J.A.; Schumacher, H.R. & Hudson A.P. (2005). Chlamydia pneumoniae and Inflammatory Arthritis, In: Chlamydia pneumoniae: Infection and disease.
Friedman H., Yamamoto Y, Bendinelli M. pp. 227-238, Ed. Kluwer Academic Publishers, ISBN 0-306-48487-0, New York.
Zhong, G.; Fan, P.; Ji, H.; Dong, F. & Huang, Y. (2001). Identification of a chlamydial protease-like activity factor responsible for the degradation of host transcription factors, J. Exp. Med. Vol. 193, No. 8, pp. 935-942.


Comments
Post a Comment