Molecular Epidemiology of Chlamydia trachomatis Urogenital Infection.
Molecular Epidemiology of Chlamydia trachomatis Urogenital Infection
1. Introduction
Each year an estimated 340 million new cases of curable sexually transmitted infections occur worldwide, with the largest proportion in the region of South and South East Asia, followed by subSaharan Africa and Latin America and the Caribbean (WHO, 2006). Chlamydia trachomatis infections are the most prevalent sexually transmitted bacteria infections recognized throughout the world. World Health Organization (WHO, 2001) estimated that there were 92 million new cases worldwide in 1999 and the incidence of infection has continued to increase each year in both industrialized and developing countries. C. trachomatis is now recognized as one the most common sexually transmissible bacterial infections among persons under than 25 years of age living in industrialized nations such as the United States, where the rate of prevalence runs at 4.2% (Miller et al., 2004).
The vast majority of published clearly, show that E, D, F and G, genotypes are isolated from urogenital tract infections with most frequency, however genotypes have yet to be consistently associated with disease severity or even disease phenotype and there is little knowledge of possible Chlamydia virulence factors, their expression and how they affect disease severity.
2. Characteristics of bacteria cell
According to the reclassification of the order Chlamydiales in 1999, the family Chlamydiaceae is now divided in two genera, Chlamydia and Chlamydophila (Everett., et al 1999).The genus Chlamydia comprises the species C. trachomatis, C. suis and C. muridarum.
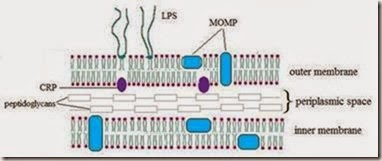
C. trachomatis are obligate intracellular parasites, possess an inner and outer membrane similar to gram-negative bacteria and a lipopolysaccharide (LPS) but do not have a peptidoglycan layer. Have many characteristics of free-living bacteria, and their metabolism follows the same general pattern; the main difference is their little capacity for generating energy. It has been shown that Chlamydiaceae are auxotrophic for ATP, GTP and UTP but not for CTP. (Tipples & McClarty, 1993).
C. tracomatis, is an exclusively human pathogen, with a tropism conjuntival and urogenital, was originally identified by their accumulation of glycogen in inclusions and their sensitivity to sulfadiazine. Based on the type of disease produced, C. trachomatis has been divided into biovars, including the lymphogranuloma venereum (LGV) biovar and the trachoma biovar, associated with human conjunctival or urogenital columnar epithelium infections. The original Wang and Grayston classification (Wang & Grayston, 1970) defined 15 C. trachomatis serovars, based on antigenic differences, designated A-K and L1-L3, which differ by the antigenicity of their major outer membrane protein (MOMP), codified by gene omp1. In addition to these serovars, numerous variants have been characterized. Serovars A, B, Ba and C, infect mainly the conjunctiva and are associated with endemic trachoma; serovars D, Da, E, F, G, Ga, H, I, J and K are predominantly isolated from the urogenital tract and are associated with sexually transmitted diseases (STD), inclusion conjunctivitis or neonatal pneumonitis in infants born to infected mothers. Serovars L1, L2, L2a and L3 can be found in the inguinal lymph nodes and are associated with LGV (Table 1).
The genome sequencing projects have shown that Chlamydia has a relatively small chromosome at between 1.04 and 1.23 Mbp and contains between 894 and 1130 predicted protein-coding genes. The fully sequence C. trachomatis genome consist of a chromosome of approximately 1.0 Mbp plus an extrachromosomal plasmid of approximately 7.5 kbp, with a total of approximately 900 likely protein-coding genes (Read et al., 2000; Carlson et al., 2005) Table 2.
The transcriptional profile of the C. trachomatis genome has been analysed by microarrays and RT-PCR (Douglas & Hatch, 2000; Shen et al., 2000). The microarrays and RT-PCR analysis has showed that 71% or 612 of the 894 genes of C. trachomatis continue to be expressed throughout the development cycle, while the others are temporally expressed (Nicholson et al., 2003). Analysis of the profiles of the temporally expressed genes has difficulties in classifying, because of the contrasting results of microarrays analysis on C. trachomatis by different groups (Belland et al., 2004; Nicholson et al., 2003).
3. The developmental cycle
C. trachomatis is a small obligate intracellular bacterium, has two developmental stages: -the extracellular elementary body (EB) and -the intracellular reticulate body (RB). EB is the infectious form metabolically inactive (EB), in this stage; the bacteria are in a state similar to that of an endospore, where the outer membrane is resistant to the environment and allows it to exist without a host cell. EB measured from 200 to 400 nm in diameter, is antigenic, non- proliferative, contains few ribosomes, is toxic in cell cultures, and is susceptible to penicillin, resistant to trypsin, osmotic shock and mechanical shock. While RB is intracellular, measured from 500 to 1500 nm in diameter, is not infective or antigenic, is proliferative, contains many ribosomes, is not toxic and is not inhibited by penicillin, is susceptible to trypsin, osmotic shock and mechanical shock.
The eukaryotic cell becomes infected when an EB adheres to the cytoplasmic membrane. The adhesion of EBs to cells is due to multiple weak specific ligand interactions, perhaps involving several molecules. There is evidence that MOMP binds to a heptaran-sulphate receptor on the host cell. The EB penetrates into the cell by endocytosis, remaining within a parasitophorous vacuole also termed inclusion or phagosome. By 2 h after infection within the phagosome EB begin differentiating into RB. Over the next several hours, RB increase in number and in size. RB can be observed dividing by binary fission by 12 h postinfection (hpi). After 18 to 24 h, the numbers of RB are maximized, and increasing numbers of RB begin differentiating back to EB, which accumulate within the lumen of the inclusion as the remainder of the RB continue to multiply. Depending on the species or strain, lysis or release from the infected cell occurs approximately 48 to 72 hpi.
4. The Infection with C. trachomatis
The C. trachomatis infects columnar epithelial cells of the ocular and urogenital mucosae. These infections have a significant impact on human health worldwide, causing trachoma, the leading cause of preventable blindness, and sexually transmitted diseases (STD) that include pelvic inflammatory disease and tubal factor infertility (Schachter, 1978; Brunham et al., 1988). Chlamydial STDs are also risk factors in cervical squamous cell carcinoma and HIV infection (Chesson & Pinkerton, 2000; Mbizvo et al., 2001).
Trachoma is one of the commonest infectious causes of blindness. The disease starts as an inflammatory infection of the eyelid and evolves to blindness due to corneal opacity. Despite long-standing control efforts, it is estimated that more than 500 million people are at high risk of infection, over 140 million persons are infected and about 6 million are blind in Africa, the Middle East, Central and South East Asia, and countries in Latin America. Trachoma is a communicable disease of families, with repeated reinfection occurring among family members. Transmission is driven by sharing of ocular secretions among young children in family or community groups, facilitated by the ubiquitous presence of flies. The disease is particularly prevalent and severe in rural populations living in poor and arid areas of the world where people have limited access to water and facial hygiene is poor. Visual loss from trachoma is 2-3-times more common in women than men and is a major cause of disability in affected communities, attacking the economically important middle- aged female population. Global elimination of trachoma as a disease of public health importance has been targeted by WHO for 2020.
The most common site of C. trachomatis infection is the urogenital tract. In men, it is the commonest cause of non-gonococcal urethritis and epididymitis however are asymptomatic in approximately 50% of men (Karam et al., 1986; Zimmerman et al., 1990). Urethritis is secondary to C. trachomatis infection in approximately 15 to 55 percent of men. Symptoms, if present, include a mild to moderate, clear to white urethral discharge. This is best observed in the morning, before the patient voids. Untreated chlamydial infection can spread to the epididymis. Patients usually have unilateral testicular pain with scrotal erythema, tenderness, or swelling over the epididymis. Men with asymptomatic infection serve as carriers of the disease, spreading the infection while only rarely suffering long-term health problems.
In women, chlamydial infection can lead to a serious reproductive morbidity. Infection of the lower genital tract occurs in the endocervix. It can cause an odorless, mucoid vaginal discharge, typically with no external pruritus. Some women develop urethritis; symptoms may consist of dysuria without frequency or urgency. Ascending infection that causes acute salpingitis with or without endometritis, also known as pelvic inflammatory disease (PID), whose long-term consequences are chronic pain, ectopic pregnancy and tubal factor infertility (Stamm, 1999). The 80% of the genital infections are asymptomatic and without clinical evidence of complications and appear to spontaneously resolve, although there only is limited knowledge about the clinical factors that influence the duration of untreated, uncomplicated genital infections (Zimmerman et al., 1990). These infections tend to be chronic and recurring and associated with scarring complications possibly related to hypersensitivity mechanisms.
A C. tracomatis infection can infect different mucosal linings, with the majority of cases in the urogenital tract but also the rectum, oropharynx and conjunctiva. Rectal chlamydial infection is often observed in men who have sex with men (Kent et al., 2005; Annan et al., 2009). Contamination of the hands with genital discharge may also lead to conjunctival infection following contact with the eyes. Babies born to mothers with infection of their genital tract frequently present with chlamydial eye infection within a week of birth (chlamydial “ophthalmia neonatorum”), and may subsequently develop pneumonia. Furthermore, an existing chlamydial infection increases the risk of contracting HIV (Joyee et al., 2005) and/or Herpes simplex infections (Freeman et al., 2006). This is especially true with the Lymphogranuloma venereum (LGV) disease, an invasive and frequently ulcerative chlamydial infection involving lymphatic tissue. LGV occurs only sporadically in North America, but it is endemic in many parts of the developing countries and represent a major risk factor for HIV acquisition (Blank et al., 2005; Schachter & Moncada, 2005; Cai et al., 2010). In addition, it was found that Chlamydial infection can be associated with human papillomavirus (Oh et al., 2009) and gonorrhea in a 20% of men and 42 % of women (Lyss et al., 2003; Srifeungfung et al., 2009).
5. Detection methods for C. trachomatis
Diagnosis of chlamydial infection is even more difficult in asymptomatic and in chronic or persistent infections where the pathogen load would be low. The large pools of asymptomatic infected people are not only at the risk of developing serious long-term sequelae but would also transmit the infection. The development of methods of detection in the laboratory highly sensitive and specific of nucleic acid amplification tests (NAATs) has been an important advance in the ability to conduct population-base screening programmes to prevent complications.
The assays that are used for diagnosis of C. trachomatis include conventional diagnostic methods and NAATs. Conventional diagnostic methods involve the isolation by cell culture and application of biochemical and immunological tests to identify. The cell culture is time consuming and laborious, and it has been in many laboratories replaced by antigen detection methods such as enzyme immunoassays (EIA), direct immunofluorescence assays (DFA) and DNA/RNA detection. EIA tests detect chlamydial LPS with a monoclonal or polyclonal antibody while DFA depending on the commercial product used detected LPS or MOMP component. DFA with a C. trachomatis-specific anti-MOMP monoclonal antibody is considered highly specific (Cles et al., 1988). DNA/RNA detection is based on the hybridization and its use is suitable for simple and fast diagnosis.
The NAATs includes polymerase chain reaction (PCR), ligase chain reaction (LCR), retrotranscription-PCR (RT-PCR) and real time-PCR. In these probes different DNA or RNA regions are used as target sequences for amplification. The major target sequences are located in cryptic plasmid, omp1 gene and rRNAs. The cryptic plasmid is present in approximately 10 copies in each C. trachomatis organism (Hatt et al., 1988), reason for which some authors suggested that amplification of C. trachomatis plasmid DNA is more sensitive (Mahony et al., 1992). However, some studies suggest that plasmid-free variants of C. trachomatis may on rare occasions be present in clinical samples (An et al., 1992). Comparative studies of the NAATs suggest that the sensitivity and specificity are quite similar, but of screening tests for C. trachomatis NAATs are more sensitive than non-NAATs (Poulakkainen et al., 1998; Ostergaard, 1999; Van Dyck et al., 2001, Black, 1997).
6. Prevalence
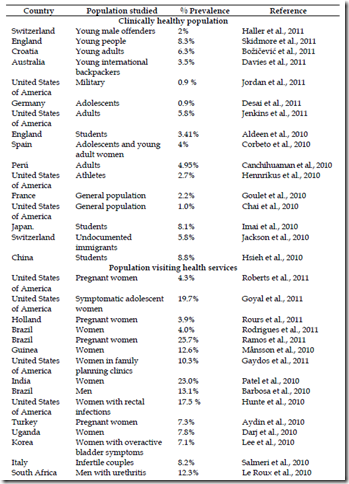
The prevalence of urogenital C. trachomatis determinate with NAATs from different parts of the world published in the present year and the 2010 is summarized in the table 3. These reports show that the prevalence is high and independent of the country, urban or rural ubication.
Studies amongst clinically healthy population have shown a prevalence rate equal or major to 4%. Two reports show lower prevalence rate of 0.9 % in United States of America (Jordan et al., 2011) and Germany (Desai et al., 2011) for population of military and adolescent students respectively, and the higher prevalence rates are for students in China with 8.8% (Hsieh et al., 2010) and young people in England with 8.3% (Skidmore et al., 2011).
The higher prevalence rate reported are for the high-risk population with average of 21.6%; that ranges from of 2.5% in Bangladesh (Huq et al., 2010) up to 72.9% in Tunesia (Znazen et al., 2010) amongst female sex workers.
7. C. trachomatis genotypes
C. trachomatis comprises distinct serogroups and serovars. Different genotyping methods are used for determination of circulating C. trachomatis serovars within a population can provide information on the epidemiology and pathogenesis of infection, including mapping sexual networks, can allow for monitoring treatment success, and may play a role in developing strategies for improved disease control, such as vaccine design.
Different genotyping methods are available to differentiate between the serovars, and are mainly based on the diversity of the omp1 gene, which encodes for the MOMP, an antigenically complex that displays serovar, serogroup, and species specificities (Baehr et al., 1988; Stephens et al., 1982). The MOMP is present in all human pathogenic Chlamydia species, contains four variable domains designated VS1, VS2, VS3, and VS4 that vary considerably between the species (Stephens et al., 1987; Yuan et al., 1989).
The genotyping methods are basically of two types: Immunological and molecular methods. The Immunological methods are based in the use of polyvalent and specific monoclonal antibodies that recognized epitopes located on the MOMP of C. trachomatis. These methods have been replaced by molecular methods, which are better in specificity and sensitivity.
The molecular methods are based in nucleic acid amplification techniques and are of two types, i) methods that analyzed the omp1 gene and ii) methods that analyzed several genes.
In methods that analyzed omp1 gene the amplication products of the omp1-PCR are analyzed by restriction fragment length polymorphism (RFLP), nucleotide sequencing, array assay and Real-Time PCR.
In RFLP technical the amplication products of the omp1-PCR are cleaved with restriction endonuclease, this test is simple, rapid and its results show a high level of agreement with the results serotyping (Morré et al., 1998) In array assay the amplication products of the omp1-PCR are analyzed by Southern blot hybridization using different DNA probes. These tests are rapid and accurately and also discriminate among multiple genotypes in one clinical specimen (Ruettger et al., 2011; Huang et al., 2008).
The nucleotide sequences of omp1 show clearly mutations, variants of omp1 and therefore providing evidence for existence of numerous subspecies. This method has a higher resolution than serotyping and RFLP (Morre et al., 1998), and has been considerate as gold standard for C. trachomatis genotyping (Sturm-Ramirez et al., 2000; Watson et al., 2002). However is still very laborious and not suitable for typing the isolates from a large number of clinical samples. A drawback is the difficulties in resolving mixed infections because peaks from different PCR products will be superposed in the chromatograms from sequencing reactions (Pedersen et al., 2009).
In genotyping by real time is evaluated with Taq Man probes in multiplex the omp-1 gene, the test is specific and convenient for the rapid routine-diagnostic with capacity to detect mixed infections.
The methods that analyzed several genes are system based on hypervariable regions identified as housekeeping genes and polymorphic membrane protein genes. These methods have showed that are capable of identifying high intraserotype variation and greater genetic diversity in comparison to use omp1 alone. Two types of methods have been described multilocus sequence typing (MLST), which analyzed candidate target regions by PCR and Sequencing (Klint et al., 2007) and the multi-locus variable number tandem repeat (VNTR) analysis and omp1 or ‘‘MLVA-omp1’’analized VNTR and omp1 sequencing together (Pedersen 2008).
8. Genotyping for C. trachomatis
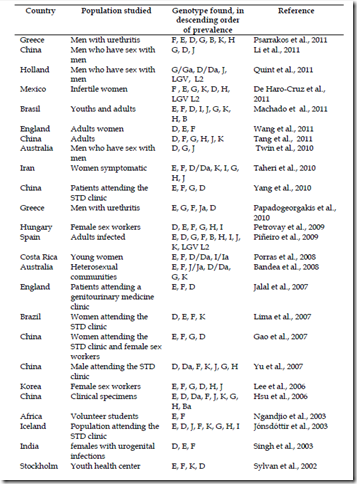
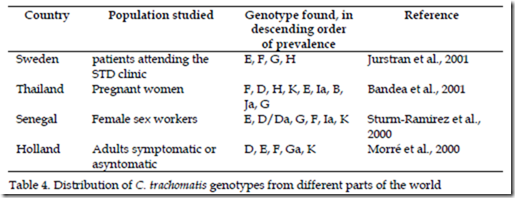
The vast majority of published data analyzed mainly with DNA sequencing of omp1 clearly, show that E, D, F and G, genotypes are isolated from urogenital tract infections with most frequency, but prevalence of individual genotypes has been reported to differ by age, sex, geographic region and racial groups as is summary in the table 4, as studies in China, Holland and Australia from men who have sex with men, which G genotype was more frequent (Li et al., 2011; Quint et al., 2011; Twin et al., 2010). Studies also have shown that nearly of 60% of all typing of clinical isolates in different parts of the world report almost five different genotypes.
However MOMP differences and genotypes have yet to be consistently associated with disease severity or even disease phenotype and there is little knowledge of possible Chlamydia virulence factors, their expression and how they affect disease severity (Byrne, 2010).
9. Conclusion
Sexually transmitted infections (STI) are responsible for human suffering and carry significant economic costs. Many STI are entirely attributable to unsafe sex. Disease burden linked to unsafe sex amounted in 2004 to 70 millions disability-adjusted life years (DALYs) worldwide, of which 52 million were accounted for by developing countries. Unsafe sex ranked second among the 10 leading risk factor causes of DALYs worldwide, and third among the leading causes of DALYs in developing countries.
Lack of education and communication are contributing factors for the increase in new cases of Chlamydia. Also, the stigma surrounding sexually transmitted disease has hindered us in limiting the spread of this disease. Since Chlamydia is such a widespread disease, more government funded educational resources should be available to assist individuals in getting information and proper medical attention. Parents also need to be responsible for communicating with their children before a problem exists. If people are properly educated, the spread of Chlamydia should decline.
10. References
Aldeen, T.; Jacobs, J.; Powell, R. (2010). Screening university students for genital chlamydial infection: another lesson to learn. Sexual Health, Vol.7, No.4, (December 2010), pp. 491-494, ISSN 1449-8987.
An, Q.; Radcliffe, G.; Vassallo, R.; Buxton, D.; O'Brien, W.; Pelletier, D.; Weisburg, W.; Klinger, J.; Olive, D. (1992). Infection with a plasmid-free variant Chlamydia related to Chlamydia trachomatis identified by using multiple assays for nucleic acid detection. Journal of Clinical Microbiology, Vol.30, No.11, (November 1992) pp. 2814- 2821, ISSN 1098-660X
Annan, N.; Sullivan, A.; Nori, A.; Naydenova, P.; Alexander, S.; McKenna, A.; Azadian, B.; Mandalia, S.; Rossi, M.; Ward, H.; Nwokolo, N. (2009). Rectal chlamydia—a reservoir of undiagnosed infection in men who have sex with men. Sexually Transmitted Infections,Vol.85, No.3, (June 2009) pp. 176-179, ISSN 1472-3263
Aydin, Y.; Atis, A.; Ocer, F.; Isenkul, R. (2010). Association of cervical infection of Chlamydia trachomatis, Ureaplasma urealyticum and Mycoplasma hominis with peritoneum colonisation in pregnancy. Journal of Obstetrics and Ginecology, Vol.30, No.8, (November 2010) pp. 809-812, ISSN 1364-6893
Baehr, W.; Zhang, Y.; Joseph, T.; Su, H.; Nano, F.; Everett, K.; Caldwell, H. (1988). Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proccedings of the National Academy of Sciences of the United States of America, Vol.85, No.11, (June 1988) pp. 4000-4004, ISSN 1091-6490
Bandea, C.; Debattista, J.; Joseph, K.; Igietseme, J.; Timms, P.; Black, C. (2008). Chlamydia trachomatis serovars among strains isolated from members of rural indigenous communities and urban populations in Australia. Journal of Clinical Microbiology, Vol.46, No.1, (January 2001) pp. 355-356, ISSN 1098-660X
Bandea, C.; Kubota, K.; Brown, T.; Kilmarx, P.; Bhullar, V.; Yanpaisarn, S.; Chaisilwattana, P.; Siriwasin, W.; Black, C. (2001). Typing of Chlamydia trachomatis strains from urine samples by amplification and sequencing the major outer membrane protein gene (omp1). Sexually Transmitted Infections, Vol.77, No.6, (December 2001) pp. 419- 422, ISSN 1472-3263
Barbosa, M.; Moherdaui, F.; Pinto, V.; Ribeiro, D.; Cleuton,M.; Miranda, A. (2010).
Prevalence of Neisseria gonorrhoeae and Chlamydia trachomatis infection in men attending STD clinics in Brazil. Revista da Sociedade Brasileira de Medicina Tropical, Vol.43, No.5, (September-October 2010), pp. 500-503, ISSN 1678-9849
Belland, R.; Ojcius, D.; Byrne, G. (2004). Chlamydia. Nature Reviews Microbiology, Vol.2, No.7,
(July 2004) pp. 530-531, ISSN 1740-1534
Black, C. (1997). Current methods of laboratory diagnosis of Chlamydia trachomatis infections.
Clinical Microbiology, Vol.10, No.1, (January 1997) pp. 160-184, ISSN 1098-6618 Blank, S.; Schillinger, J.; Harbatkin, D. (2005). Lymphogranuloma venereum in the
industrialised world. Lancet, Vol.365, No.9471, (May 2005) pp. 1607-1608, ISSN 1474-547X Božičević, I.; Grgić, I.; Židovec-Lepej, S.; Čakalo, J.; Belak-Kovačević, S.; Štulhofer, A.;
Begovac, J. (2011). Urine-based testing for Chlamydia trachomatis among young adults in a population-based survey in Croatia: feasibility and prevalence. BMC Public Health, Vol.14, No.11, (April) pp.230, ISSN 1471-2458 Brunham, R.; Binns, B.; Guijon, F.; Danforth, D.; Kosseim, M.; Rand, F.; McDowell, J.;
Rayner, E. (1988). Etiology and outcome of acute pelvic inflammatory disease. The Journal of Infectious Diseases, Vol.158, No.3, (September 1988) pp. 510-517, ISSN 1537-6613
Byrne G. (2010). Chlamydia trachomatis strains and virulence: rethinking links to infection prevalence and disease severity. The Journal of Infectious Diseases, Vol.201, Suppl No.2:, (January 2010) pp. S126-S133, ISSN 1537-6613
Cai, L.; Kong, F.; Toi, C.; van Hal, S.; Gilber, G. (2010). Differentiation of Chlamydia trachomatis lymphogranuloma venereum-related serovars from other serovars using multiplex allele-specific polymerase chain reaction and high-resolution melting analysis. International Journal of STD &AIDS, Vol.21, No.11, (February 2010) pp. 101-104, ISSN 1758-1052
Canchihuaman, F.; Carcamo, C.; Garcia, P.; Aral S.; Whittington, W.; Hawes, S.; Hughes, J.; Holmes, K. (2010). Non-monogamy and risk of infection with Chlamydia trachomatis and Trichomonas vaginalis among young adults and their cohabiting partners in Peru. Sexually transmitted Infections, Vol.86, Suppl No.3, (December 2010), pp. 3:iii37-3:iii44, ISSN 1472-3263
Carlson, J.; Porcella, S.; McClarty, G.; Caldwell, H. (2005). Comparative genomic analysis of Chlamydia trachomatis oculotropic and genitotropic strains. Infection and Immunity, Vol.73, No.10, (October 2005) pp. 6407-6418, ISSN 1098-5522
Chai, S.; Aumakhan, B.; Barnes, M.; Jett-Goheen, M.; Quinn, N.; Agreda, P.; Whittle, P.; Hogan, T.; Jenkins, W.; Rietmeijer, C.; Gaydos C. (2010). Internet-based screening for sexually transmitted infections to reach nonclinic populations in the community: risk factors for infection in men. Sexually Transmitted Diseases, Vol.37, No.12, (December 2010) pp. 756-763, ISSN 1537-4521
Chesson, H. & Pinkerton, S. (2000). Sexually transmitted diseases and the increased risk for HIV transmission: implications for cost-effectiveness analyses of sexually transmitted disease prevention interventions. Journal of Acquired Immune Deficiency Syndromes, Vol.24, No.1, (May 2000) pp. 48-56, ISSN 1944-7884
Chkhartishvili, N.; Dvali, N.; Khechiashvili, G.; Sharvadze, L.; Tsertsvadze, T. (2010). High seroprevalence of Chlamydia trachomatis in newly diagnosed human immunodeficiency virus patients in georgia. Georgian Medical News, Vol.189, (December 2010), pp. 12-16, ISSN 1512-0112
Cles, L.; Bruch, K.; Stamm, W. (1988). Staining characteristics of six commercially available monoclonal immunofluorescence reagents for direct diagnosis of Chlamydia trachomatis infections. Journal of Clinical Microbiology, Vol.26, No.9, (September 1988) pp. 1735-1737, ISSN 1098-660X
Corbeto, E.; Lugo, R.; Martró, E.; Falguera, G.; Ros, R.; Avecilla, A.; Coll, C.; Saludes, V.; Casabona, J. (2010). Epidemiological features and determinants for Chlamydia trachomatis infection among women in Catalonia, Spain. International Journal of STD
&AIDS, Vol.21, No.10, (October 2010) pp. 718-722, ISSN 1758-1052
Darj, E.; Mirembe, F.; Råssjö, E. (2010). STI-prevalence and differences in social background and sexual behavior among urban and rural young women in Uganda. Sexual & Reproductive Healthcare, Vol.1, No.3, (August 2010), pp. 111-115, ISSN 1877-5764
Davies, S.; Karagiannis, T.; Headon, V.; Wiig, R.; Duffy, J. (2011). Prevalence of genital
chlamydial infection among a community sample of young international backpackers in Sydney, Australia. International Journal of STD &AIDS, Vol.22, No.3, (March 2011) pp. 160-164, ISSN 1758-1052
Dean, D.; Bruno, W.; Wan, R.; Gomes, J.; Devignot, S.; Mehari, T.; de Vries, H.; Morré, S.; Myers, G.; Read, T.; Spratt, B. (2009). Predicting phenotype and emerging strains among Chlamydia trachomatis infections. Emerging Infectious Diseases, Vol.15, No.9, (September 2009) pp. 1385-1394, ISSN 1080-6059
De Haro-Cruz, M.; Deleón-Rodríguez, I.; Escobedo-Guerra, M.; López-Hurtado, M.; Arteaga –Troncoso, G.; Ortiz-Ibarra. F.; Guerra-Infante, F. (2011). Genotyping of Chlamydia trachomatis from endocervical specimens of infertile Mexican women. Enfermedades Infecciosas y Microbiologia Clinica, Vol.29, No.2, (February 2011), pp. 102-108, ISSN 1578-1852
Desai, S.; Meyer, T.; Thamm, M.; Hamouda, O.; Bremer, V. (2011 ). Prevalence of Chlamydia trachomatis among young German adolescents, 2005-06. Sexual Health, Vol.8, No.1, pp. 120-122, ISSN 1449-8987
Douglas, A. & Hatch, T. (2000). Expression of the transcripts of the sigma factors and putative sigma factor regulators of Chlamydia trachomatis L2. Gene, Vol.247, No.1-2, (April 2000) pp. 209-214, ISSN 1879-0038
Everett, K.; Bush, R.; Andersen, A. (1999). Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. International Journal of Systematic Bacteriology, Vol.49, Pt No.2, (April 1999) pp. 415-440, ISSN 0020-7713
Folch, C.; Casabona, J.; Brugal, M.; Majó, X.; Esteve, A.; Meroño, M.; Gonzalez, V. (2011).
Sexually Transmitted Infections and Sexual Practices among Injecting Drug Users in Harm Reduction Centers in Catalonia. European Addiction Research, Vol.17, No.5, (July 2011) pp. 271-278, ISSN 1421-9891
Freeman, E.; Weiss, H.; Glynn, J.; Cross, P.; Whitworth, J.; Hayes, R. (2006). Herpes simplex
virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Acquired immune deficiency syndrome, Vol.20, No.1, (January 2006) pp. 73-83, ISSN 1473-5571
Fresse, A.; Sueur, J.; Hamdad, F. (2010). Diagnosis and follow-up of genital chlamydial
infection by direct methods and by detection of serum IgG, IgA and secretory IgA. Indial Journal of Medical Microbiology, Vol.28, No.4, (October-December 2010), pp. 326-33144, ISSN 1998-3646
Gao, X.; Chen, X.; Yin, Y.; Zhong, M.; Shi, M.; Wei, W.; Chen, Q.; Peeling, R.; Mabey, D. (2007). Distribution study of Chlamydia trachomatis serovars among high-risk women in China performed using PCR-restriction fragment length polymorphism genotyping. Journal of Clinica Microbiology, Vol.45, No.4, (February 2007) pp. 1185- 1189, ISSN 1098-660X
Gaydos, C.; Barnes, M.; Aumakhan, B.; Quinn, N.; Wright, C.; Agreda, P.; Whittle, P.; Hogan T. (2011). Chlamydia trachomatis age-specific prevalence in women who used an internet-based self-screening program compared to women who were screened in family planning clinics. Sexually Transmitted Diseases, Vol.38, No.2, (February 2011), pp. 74-78, ISSN 1537-4521
Goulet, V.; de Barbeyrac, B.; Raherison, S.; Prudhomme, M.; Semaille, C.; Warszawski, J.;
CSF group. (2010). Prevalence of Chlamydia trachomatis: results from the first national population-based survey in France. Sexual Transmitted Infections, Vol.86, No.4, (August 2010) pp. 263-270, ISSN 1472-3263
Goyal, M.; Hayes, K.; McGowan, K.; Fein, J.; Mollen C. (2011). Prevalence of Trichomonas
vaginalis Infection in Symptomatic Adolescent Females Presenting to a Pediatric Emergency Department. Academic Emergency Medicine , Vol.18, No.7, (July 1990) pp. 763-766, ISSN 1553-2712
Haller, D.; Steiner, A.; Sebo, P.; Gaspoz, J.; Wolff, H. (2011). Chlamydia trachomatis infection in males in a juvenile detention facility in Switzerland. Swiss Medical Weekly, Vol.141, (July 2011), ISSN 1424-3997
Hatt, C.; Ward, M.; Clarke, I. (1988). Analysis of the entire nucleotide sequence of the cryptic plasmid of Chlamydia trachomatis serovar L1. Evidence for involvement in DNA replication. Nucleic Acids Research, Vol.16, No.9, (May 1988) pp. 4053-4067, ISSN 1362-4962
Hennrikus, E.; Oberto, D.; Linder, J.; Rempel, J.; Hennrikus, N. (2010). Sports preparticipation examination to screen college athletes for Chlamydia trachomatis. Medicine and Sciece in Sports and Exercise, Vol.42, No.4, (April 2010), pp. 683-688, ISSN 1530-0315
Hsieh, Y.; Shih, T.; Lin, H.; Hsieh, T.; Kuo, M.; Lin, C.; Gaydos, C. (2010). High-risk sexual behaviours and genital chlamydial infections in high school students in Southern Taiwan. International Journal of STD &AIDS, Vol.21, No.4, (April 2010) pp. 253-259, ISSN 1758-1052
Hsu, M.; Tsai, P.; Chen, K.; Li, L.; Chiang, C.; Tsai, J.; Ke, L.; Chen, H.; Li, S. (2006).
Genotyping of Chlamydia trachomatis from clinical specimens in Taiwan. Journal of Medical Microbiology, Vol.55, Pt No.3, (March 2006) pp. 301-308, ISSN 1473-5644
Huang, C.; Wong, W.; Li, L.; Chang, C.; Chen, B.; Li, S. (2008). Genotyping of Chlamydia trachomatis by microsphere suspension array. Journal of Clinical Microbiology, Vol.46, No.3, (Mar 2008), pp. 1126-1128, ISSN 1098-660X
Hunte, T.; Alcaide, M.; Castro, J. (2010). Rectal infections with chlamydia and gonorrhoea in women attending a multiethnic sexually transmitted diseases urban clinic. International Journal of STD &AIDS, Vol.21, No.12, (December 2010) pp. 819-822, ISSN 1758-1052
Huq, M.; Chawdhury, F.; Mitra, D.; Islam, M.; Salahuddin, G.; Das, J.; Rahman, M. (2010). A pilot study on the prevalence of sexually transmitted infections among clients of brothel-based female sex workers in Jessore, Bangladesh. International Journal of STD &AIDS, Vol.21, No.4, (April 2010) pp. 300-301, ISSN 1758-1052
Imai, H.; Nakao, H.; Shinohara, H.; Fujii, Y.; Tsukino, H.; Hamasuna, R.; Osada, Y.; Fukushima, K.; Inamori, M.; Ikenoue, T.; Katoh T. (2010). Population-based study of asymptomatic infection with Chlamydia trachomatis among International Journal of STD &AIDS, Vol.21, No.5, (May 2010) pp. 362-366, ISSN 1758-1052
Jackson, Y.; Sebo, P.; Aeby, G.; Bovier, P.; Ninet, B.; Schrenzel, J.; Sudre, P.; Haller, D.; Gaspoz, J.; Wolff, H. (2010). Prevalence and associated factors for Chlamydia trachomatis infection among undocumented immigrants in a primary care facility in Geneva, Switzerland: a cross-sectional study. Journal of Immigrant and Minority Health, Vol.12, No.6, (December 2010) pp. 909-9144, ISSN 1557-1920
Jalal, H.; Stephen, H.; Bibby, D.; Sonnex, C.; Carne, C. (2007). Molecular epidemiology of genital human papillomavirus and Chlamydia trachomatis among patients attending a genitourinary medicine clinic - will vaccines protect? International Journal of STD
&AIDS, Vol.18, No.9, (September 2010) pp. 617-621, ISSN 1758-1052
Jenkins, W.; Rabins, C.; Barnes, M.; Agreda, P.; Gaydos, C. (2011). Use of the internet and self-collected samples as a sexually transmissible infection intervention in rural Illinois communities. Sexual Health, Vol.8, No.1, (March 2011), pp. 79-85, ISSN 1449- 8987
Jin, X.; Chan, S.; Ding, G.; Wang, H.; Xu, J.; Wang, G.; Chang, D.; Reilly, K.; Wang, N. (2011).
Prevalence and risk behaviours for Chlamydia trachomatis and Neisseria gonorrhoeae infection among female sex workers in an HIV/AIDS high-risk area. International Journal of STD &AIDS, Vol.22, No.2, (February 2011) pp. 80-84, ISSN 1758-1052
Jo, S.; Shin, J.; Song, K.; Kim, J.; Hwang, K.; Bhally H. (2011). Prevalence and Correlated Factors of Sexually Transmitted Diseases-Chlamydia, Neisseria, Cytomegalovirus-in Female Rape Victims. The Journal of Sexual Medicine, Vol.8, No.8, (August 2011) pp. 2317-2326, ISSN 1743-6109
Jónsdóttir, K.; Kristjánsson, M.; Hjaltalín, O.; Steingrímsson, O. (2003). The molecular epidemiology of genital Chlamydia trachomatis in the greater Reykjavik area, Iceland. Sexually transmitted diseases, Vol.30, No.3, (March 2003), pp. 249-256, ISSN 1537-4521
Jordan, N.; Lee, S.; Nowak, G.; Johns, N.; Gaydos, J. (2011). Chlamydia trachomatis reported
among U.S. active duty service members, 2000-2008. Military Medicine, Vol.176, No.3, (March 2011), pp. 312-319, ISSN 1930-613X
Joyee, A.; Thyagarajan, S.; Reddy, E.; Venkatesan, C.; Ganapathy, M. (2005). Genital chlamydial infection in STD patients: its relation to HIV infection. Indian Journal of Medical Microbiology, Vol.23, No.1, (January 2005) pp. 37-40, ISSN 1998-3646
Jurstrand, M.; Falk,L.; Fredlund, H.; Lindberg, M.; Olcén, P.; Andersson, S.; Persson, K.; Albert, J.; Bäckman, A. (2001). Characterization of Chlamydia trachomatis omp1 genotypes among sexually transmitted disease patients in Sweden. Journal of Clinical Microbiology, Vol.39, No.11, (November 2001), pp. 3915-3919, ISSN 1098- 660X
Karam, G.; Martin, D.; Flotte, T.; Bonnarens, F.; Joseph, J.; Mroczkowski, T.; Johnson, W. (1986). Asymptomatic Chlamydia trachomatis infections among sexually active men. (1986). The Journal of Infectious Diseases, Vol.154, No.5, (November 1986) pp.900-903, ISSN 1537-6613
Kent, C.; Chaw, J.; Wong, W.; Liska, S.; Gibson, S.; Hubbard, G.; Klausner, J. (2005).
Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clinical Infectious Diseases, Vol.41, No.1, (July 2005) pp. 67-74, ISSN 1537-6591
Khan, M.; Unemo, M.; Zaman, S.; Lundborg, C. (2011). HIV, STI prevalence and risk behaviours among women selling sex in Lahore, Pakistan. BMC Infectious Diseases, Vol.11, No.1, (May 2011), pp. 119, ISSN 1471-2334
Klint, M.; Fuxelius, H.; Goldkuhl, R.; Skarin, H.; Rutemark, C.; Andersson, S.; Persson, K.; Herrmann, B. (2007). High-resolution genotyping of Chlamydia trachomatis strains by multilocus sequence analysis. Journal of Clinical Microbiology, Vol.45, No.5, (May 2007), pp. 1410-1414, ISSN 1098-660X
Kwena, Z.; Bukusi, E.; Ng'ayo, M.; Buffardi, A.; Nguti, R.; Richardson, B.; Sang, N.; Holmes
K. (2010). Prevalence and risk factors for sexually transmitted infections in a high-
risk occupational group: the case of fishermen along Lake Victoria in Kisumu, Kenya. International Journal of STD &AIDS, Vol.21, No.10, (October 2010) pp. 708- 713, ISSN 1758-1052
Lee, G.; Park,J.; Kim, S.; Yoo, C.; Seong, W. (2006). OmpA genotyping of Chlamydia trachomatis from Korean female sex workers. The Journal of Infection, Vol.52, No.6, (June 2006), pp. 451-454, ISSN 1532-2742
Lee, J.; Jung, S.; Kwon, D.; Jung, M.; Park, B. (2010). Condom Use and Prevalence of Genital Chlamydia trachomatis Among the Korean Female Sex Workers. Epidemiology and Health, Vol.32, (August 2010) pp. e2010008, ISSN 2092-7193
Lee, Y.; Kim, J.; Kim, J.; Park, W.; Choo, M.; Lee, K. (2010). Prevalence and treatment efficacy of genitourinary mycoplasmas in women with overactive bladder symptoms. Korean Journal of Urology, Vol.51, No.9, (September 2010) pp. 625-630, ISSN 2005- 6745
Le Roux, M.; Ramoncha, M.; Adam, A.; Hoosen A. (2010). A etiological agents of urethritis in symptomatic South African men attending a family practice. International Journal of STD &AIDS, Vol.21, No.7, (July 2010) pp. 477-481, ISSN 1758-1052
Li, J.; Cai, Y.; Yin, Y.; Hong, F.; Shi, M.; Feng, T.; Peng, R.; Wang, B.; Chen, X. (2011).
Prevalence of anorectal Chlamydia trachomatis infection and its genotype distribution among men who have sex with men in Shenzhen, China. Japanese Journal of Infectious Diseases, Vol.64, No.2, pp. 143-146, ISSN 1344-6304
Lima, H.; Oliveira, M.; Valente, B.; Afonso, D.; Darocha, W.; Souza, M.; Alvim, T.; Barbosa- Stancioli, E.; Noronha, F. (2007). Genotyping of Chlamydia trachomatis from endocervical specimens in Brazil. Sexually transmitted diseases, Vol.34, No.9, (September 2007), pp. 709-717, ISSN 1537-4521
Lyss, S.; Kamb, M.; Peterman, T.; Moran, J.; Newman, D.; Bolan, G.; Douglas, J.; Iatesta, M.; Malotte, C.; Zenilman, J.; Ehret, J.; Gaydos, C.; Newhall, W. Project RESPECT Study Group. (2003). Chlamydia trachomatis among patients infected with and treated for Neisseria gonorrhoeae in sexually transmitted disease clinics in the United States. Annals of Internal Medicine, Vol.139, No.3, ( August 2003) pp. 178-185, ISSN 1539- 3704
Machado, A.; Bandea, C.; Alves, M.; Joseph, K.; Igietseme, J.; Miranda, A.; Guimarães, E.; Turchi, M.; Black, C. (2011). Distribution of Chlamydia trachomatis genovars among youths and adults in Brazil. Journal of Medical Microbiology, Vol.60, Pt No.4, (April 2011), pp. 472-476, ISSN 1473-5644
Mahony, J.; Luinstra, K.; Sellors, J; Jang, D.; Chernesky MA. (1992). Confirmatory polymerase chain reaction testing for Chlamydia trachomatis in first-void urine from asymptomatic and symptomatic men. Journal of Clinical Microbiology, Vol.30, No.9, (September 1992) pp. 2241-2245, ISSN 1098-660X
Månsson, F.; Camara, C.; Biai, A.; Monteiro, M.; da Silva Z.; Dias, F.; Alves, A.; Andersson, S.; Fenyö, E.; Norrgren, H.; Unemo, M. (2010). High prevalence of HIV-1, HIV-2 and other sexually transmitted infections among women attending two sexual health clinics in Bissau, Guinea-Bissau, West Africa. International Journal of STD
&AIDS, Vol.21, No.9, (September 2010) pp. 631, ISSN 1758-1052
Mawu, F.; Davies, S.; McKechnie, M.; Sedyaningsih, E.; Widihastuti, A.; Hillman, R. (2011).
Sexually transmissible infections among female sex workers in Manado, Indonesia, using a multiplex polymerase chain reaction-based reverse line blot assay. Sexual Health, Vol.8, No.1, (March 2011) pp. 52-60, ISSN 1449-8987
Mbizvo, E.; Msuya, S.; Stray-Pedersen, B.; Sundby, J.; Chirenje, M.; Hussain, A. (2001). HIV seroprevalence and its associations with the other reproductive tract infections in asymptomatic women in Harare, Zimbabwe. International Journal of STD &AIDS, Vol.12, No.8, (August 2001) pp.524-531, ISSN 1758-1052
Miller, W.; Ford, C.; Morris, M.; Handcock, M.; Schmitz, J.; Hobbs, M.; Cohen, M.; Harris, K.; Udry, J. (2004). Prevalence of chlamydial and gonococcal infections among young adults in the United States. The Journal of the American Medical Association, Vol.291, No.18, (May 2004) pp. 2229-2236, ISSN 1538-3598
Morré, S.; Meijer, C.; Munk, C.; Krüger-Kjaer, S.; Winther, J.; Jørgensens, H.; van Den Brule, A. (2000). Pooling of urine specimens for detection of asymptomatic Chlamydia trachomatis infections by PCR in a low-prevalence population: cost-saving strategy for epidemiological studies and screening programs. Journal of Clinical Microbiology, Vol.38, No.4, (April 2000) pp. 1679-1680, ISSN 1098-660X
Morré, S.; Ossewaarde, J.; Lan, J.; van Doornum, G.; Walboomers, J.; MacLaren, D.; Meijer, C.; van den Brule, A. (1998). Serotyping and genotyping of genital Chlamydia trachomatis isolates reveal variants of serovars Ba, G, and J as confirmed by omp1 nucleotide sequence analysis. Journal of Clinical Microbiology, Vol.36, No.2, (February 1998) pp. 345-351, ISSN 1098-660X
Ngandjio, A.; Clerc, M.; Fonkoua, M.; Thonnon, J.; Njock, F.; Pouillot, R.; Lunel, F.; Bebear, C.; De Barbeyrac, B.; Bianchi, A. (2003). Screening of volunteer students in Yaounde (Cameroon, Central Africa) for Chlamydia trachomatis infection and genotyping of isolated C. trachomatis strains. Journal of Clinical Microbiology, Vol.41, No.9, (September 2003), pp. 4404-4407, ISSN 1098-660X
Nicholson, T.; Olinger, L.; Chong, K.; Schoolnik, G.; Stephens R. (2003). Global stage-specific gene regulation during the developmental cycle of Chlamydia trachomatis. Journal of Bacteriology, Vol.185, No.10, (May 2010) pp. 3179-3189, ISSN 1098-5530
Oh, J.; Franceschi, S.; Kim, B.; Kim, J.; Ju, Y.; Hong, E.; Chang, Y.; Rha, S.; Kim, H.; Kim, J.; Kim, C.; Shin, H. (2009). Prevalence of human papillomavirus and Chlamydia trachomatis infection among women attending cervical cancer screening in the Republic of Korea. European Journal Cancer Prevention, Vol.18, No.1, (February 2009), pp. 56-61, ISSN 1473-5709
Ostergaard L. (1999). Diagnosis of urogenital Chlamydia trachomatis infection by use of DNA amplification. Acta pathologica, microbiologica, et immunologica Scandinavica, Vol.89, No.5, pp. 36, ISSN 1362-4962
Papadogeorgakis, H.; Pittaras, T.; Papaparaskevas, J.; Pitiriga, V.; Katsambas, A.; Tsakris A. (2010). Chlamydia trachomatis serovar distribution and Neisseria gonorrhoeae coinfection in male patients with urethritis in Greece. Journal of Clinical Microbiology, Vol.48, No.6, (June 2001) pp. 2231-2234, ISSN 1098-660X
Patel, A.; Sachdev, D.; Nagpal, P.; Chaudhry, U.; Sonkar, S.; Mendiratta, S.; Saluja D. (2011).
Prevalence of Chlamydia infection among women visiting a gynaecology outpatient department: evaluation of an in-house PCR assay for detection of Chlamydia trachomatis. Annals of Clinical Microbiology and Antimicrobials, Vol.9, No.24, (September 2011), ISSN 1476-0711
Pedersen, L.; Herrmann, B.; Moller, J.; (2009). Typing Chlamydia trachomatis: from egg yolk to nanotechnology. FEMS immunology and medical microbiology, Vol.55, No.2, (March 2009), pp. 120-130, ISSN 1574-695X
Pedersen, L.; Podenphant, L.; Moller, J. (2008). Highly discriminative genotyping of Chlamydia trachomatis using omp1 and a set of variable number tandem repeats. Clinical Microbiology and Infection, Vol.14, No.7, (July 2008), pp. 644-652, ISSN 1469-0691
Petrovay, F.; Balla, E.; Németh, I.; Gönczöl, E. (2009). Genotyping of Chlamydia trachomatis from the endocervical specimens of high-risk women in Hungary. Journal of Medical Microbiology, Vol.58, Pt No.6, (June 2010), pp. 760-764, ISSN 1473-5644
Piñeiro, L.; Montes, M.; Gil-SetasA.; Camino X.; Echeverria.; Cilla G. (2009). Genotyping of Chlamydia trachomatis in an area of northern Spain. Enfermedades Infecciosas y Microbiologia Clinica, Vol.27, No.8, (October 2009), pp. 462-464, ISSN 1578-1852
Platt, L.; Grenfell, P.; Bonell, C.; Creighton, S.; Wellings, K.; Parry, J.; Rhodes T. (2011). Risk of sexually transmitted infections and violence among indoor-working female sex workers in London: the effect of migration from Eastern Europe. Sexually transmitted Infections, Vol.86, No.5, (August 2011), pp. 377-384, ISSN 1472-3263
Porras, C.; Safaeian, M.; González, P.; Hildesheim, A.; Silva, S.; Schiffman, M.; Rodríguez, A.; Wacholder, S.; Freer, E.; Quint, K.; Bratti, C.; Espinoza, A.; Cortes, B.; Herrero, R.; Costa Rica HPV Vaccine Trial (CVT) Group. (2008). Epidemiology of genital Chlamydia trachomatis infection among young women in Costa Rica. Sexually transmitted diseases, Vol.35, No.5, (May 2008), pp. 461-468, ISSN 1537-4521
Psarrakos, P.; Papadogeorgakis, E.; Sachse, K.; Vretou, E. (2011). Chlamydia trachomatis ompA genotypes in male patients with urethritis in Greece - Conservation of the serovar distribution and evidence for mixed infections with Chlamydophila abortus. Molecular and Cellular Probes, Vol.25, No.1, (August 2011), pp. 168-173, ISSN 1096-1194
Puolakkainen, M.; Hiltunen-Back, E.; Reunala, T.; Suhonen, S.; Lähteenmäki, P.; Lehtinen, M.; Paavonen, J. (1998). Comparison of performances of two commercially available tests, a PCR assay and a ligase chain reaction test, in detection of urogenital Chlamydia trachomatis infection. Journal of Clinical Microbiology, Vol.36, No.6, (June 1998) pp. 1489-1493, ISSN 1098-660X
Quint, K.; Bom, R.; Quint, W.; Bruisten, S.; van der Loeff, M.; Morré S.; de Vries, H. (2011).
Anal infections with concomitant Chlamydia trachomatis genotypes among men who have sex with men in Amsterdam, the Netherlands. Sexual Transmitted BMC Infectious Diseases, Vol.11, No.63, (March 2011), ISSN 1471-2334
Ramos, B.; Polettini, J.; Marcolino, L.; Vieira, E.; Marques, M.; Tristão, A.; Nunes, H.; Rudge, M.; Silva, M. (2011). Prevalence and risk factors of Chlamydia trachomatis cervicitis in pregnant women at the genital tract infection in obstetrics unit care at Botucatu Medical School, São Paulo State University-UNESP, Brazil. Journal Low Genital Trac Disease, Vol.15, No.1, (January 2011) pp. 20-24, ISSN 1526-0976
Read, T.; Brunham, R.; Shen, C.; Gill, S.; Heidelberg, J.; White, O.; Hickey, E.; Peterson, J.; Utterback, T.; Berry,K.; Bass, S.; Linher, K.; Weidman, J.; Khouri, H.; Craven, B.; Bowman, C.; Dodson, R.; Gwinn, M.; Nelson, W.; DeBoy, R.; Kolonay, J.; McClarty, G.; Salzberg, S.; Eisen, J.; Fraser, C. (2000). Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Research, Vol.28, No.6, (March 2000) pp. 1397-1406, ISSN 1362-4962
Roberts, S.; Sheffield, J.; McIntire, D.; Alexander, J. (2011). Urine screening for Chlamydia trachomatis during pregnancy. Obstetrics Gynecology. Military Medicine, Vol.117, No.4, (April 2011), pp. 883-885, ISSN 1873-233X
Rodrigues, M.; Fernandes, P.; Haddad, J.; Paiva, M.; Souza, Mdo.; Andrade, T.; Fernandes A. (2011). Frequency of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Mycoplasma hominis and Ureaplasma species in cervical samples. Journal of Obstetrics and Ginecology, Vol.31, No.3, pp. 237-241, ISSN 1364-6893
Rours, G.; Duijts, L.; Moll, H.; Arends, L.; de Groot, R,; Jaddoe, V.; Hofman, A.; Steegers, E.; Mackenbach, J.; Ott, A.; Willemse, H.; van der Zwaan, E.; Verkooijen, R.; Verbrugh, H. (2011). Chlamydia trachomatis infection during pregnancy associated with preterm delivery: a population-based prospective cohort study. European Journal of Epidemiology, Vol.26, No.6, (May 2011) pp. 493-502, ISSN 1573-7284
Ruettger, A.; Feige, J.; Slickers, P.; Schubert, E.; Morré, S.; Pannekoek, Y.; Herrmann, B.; de Vries, H.; Ehricht, R.; Sachse, K. (2011). Genotyping of Chlamydia trachomatis strains from culture and clinical samples using an ompA-based DNA microarray assay. Molecular and Cellular, Vol.25, No.1, (February 2011), pp. 19-27, ISSN 1096-1194
Salmeri, M.; Santanocita, A.; Toscano, M.; Morello, A.; Valenti, D.; La Vignera, S.; Bellanca, S.; Vicari, E.; Calogero A. (2010). Chlamydia trachomatis prevalence in unselected infertile couples. Systems Biology in Reproductive Medicine, Vol.56, No.6, (December 2010) pp. 450-456, ISSN 1939-6376
Schachter, J. (1978). Chlamydial infections (first of three parts). The New England Journal Medicine., Vol.298, No.8, (February 1978) pp. 428-435, ISSN 1533-4406
Schachter, J. & Moncada J. (2005). Lymphogranuloma venereum: how to turn an endemic disease into an outbreak of a new disease? Start looking. Sexually Transmitted Diseases, Vol.32, No.6, (June 2005) pp. 331-332, ISSN 1537-4521
Shen, L.; Shi, Y.; Douglas, A.; Hatch, T.; O'Connell, C.; Chen, J.; Zhang, Y. (2000).
Identification and characterization of promoters regulating tuf expression in Chlamydia trachomatis serovar F. Archives of Biochemimistry and Biophysics, Vol.379, No.1, (July 2000) pp. 46-56, ISSN 1096-0384 Silitonga, N.; Davies, S.; Kaldor, J.; Wignall, S.; Okoseray, M. (2011). Prevalence over time and risk factors for sexually transmissible infections among newly-arrived female sex workers in Timika, Indonesia. Sexual Health, Vol.8, No.1, (March 2011) pp. 61- 64, ISSN 1449-8987
Singh, V.; Salhan, S.; Das, B.; Mittal, A. (2003). Predominance of Chlamydia trachomatis serovars associated with urogenital infections in females in New Delhi, India. Journal of Clinical Microbiology, Vol.41, No.6, (June 2003), pp. 2700-2702, ISSN 1098- 660X
Skidmore, S.; Copley, S.; Cordwell, D.; Donaldson, D.; Ritchie, D.; Spraggon, M. (2011).
Positive nucleic acid amplification tests for Neisseria gonorrhoeae in young people tested as part of the National Chlamydia Screening Programme. International Journal of STD &AIDS, Vol.22, No.7, (July 2010) pp. 398-399, ISSN 1758-1052
Srifeungfung, S.; Roongpisuthipong, A.; Asavapiriyanont, S.; Lolekha, R.;
Tribuddharat,C.; Lokpichart, S.; Sungthong, P.; Tongtep, P. (2009). Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae in HIV-seropositive patients and gonococcal antimicrobial susceptibility: an update in Thailand. Japanese Journal of Infectious Diseases, Vol.62, No.6, (November 2009), pp. 467-470, ISSN 1344-6304
Sylvan, S.; Von Krogh, G.; Tiveljung, A.; Siwerth, B.; Henriksson, L.; Norén, L.; Asp, A.; Grillner, L. (2002). Screening and genotyping of genital Chlamydia trachomatis in urine specimens from male and female clients of youth-health centers in Stockholm County. Sexually Transmitted Diseases, Vol.29, No.7, (July 2002), pp. 379-386, ISSN 1537-4521
Stamm, W. (1999). Chlamydia trachomatis is infections of the adult. In sexually transmitted
disease 3rd edition. Edited by: Holmes KK, Sparling PF, Mardh PA. McGraw-Hill , 407-422, ISBN 978-0070296886 New York, United States of America.
Steiner, A.; Haller, D.; Elger, B.; Sebo, P.; Gaspoz, J.; Wolff, H. (2010). Chlamydia trachomatis infection in a Swiss prison: a cross sectional study. Swiss Medical Weekly, Vol.140, (November 2010) pp. w13126, ISSN 1424-3997
Stephens, R.; Sanchez-Pescador, R.; Wagar E.; Inouye, C.; Urdea M. (1987). Diversity of Chlamydia trachomatis major outer membrane protein genes. Journal of Bacteriology, Vol.169, No.9, (September 1987) pp. 3879-3885, ISSN 1098-5530
Stephens R.; Tam, M.; Kuo, C.; Nowinski, R. (1982). Monoclonal antibodies to Chlamydia trachomatis: antibody specificities and antigen characterization. Journal of Immunity, Vol.128, No.3, (March 1982) pp. 1083-1089, ISSN 1550-6606
Sturm-Ramirez, K.; Brumblay, H.; Diop, K.; Guèye-Ndiaye, A.; Sankalé, J.; Thior, I.; N'Doye, I.; Hsieh, C.; Mboup, S.; Kanki, P. (2000). Molecular epidemiology of genital Chlamydia trachomatis infection in high-risk women in Senegal, West Africa. Journal of Clinical Microbiology, Vol.38, No.1, (January 2000) pp. 138-145, ISSN 1098-660X
Taheri, B.; Motamedi, H.; Ardakani, M. (2010). Genotyping of the prevalent Chlamydia trachomatis strains involved in cervical infections in women in Ahvaz, Iran. Journal of Medical Microbiology, Vol.59, Pt No.9, (September 2010), pp. 1023-1028, ISSN 1473- 5644
Tang, J.; Zhou, L.; Liu, X.; Zhang, Ch.; Zhao, Y.; Wang Y. (2011). Novel multiplex real-time PCR system using the SNP technology for the simultaneous diagnosis of Chlamydia trachomatis, Ureaplasma parvum and Ureaplasma urealyticum and genetic typing of serovars of C. trachomatis and U. parvum in NGU. Molecular and Cellular Probes, Vol.25, No.1, (February 2011), pp. 55-59, ISSN 1096-1194
Tanudyaya, F.; Rahardjo, E.; Bollen, L.; Madjid, N.; Daili, S.; Priohutomo, S.; Morineau, G.; Nurjannah, Roselinda, Anartati A.; Purnamawati, K.; Mamahit, E. (2010).
Prevalence of sexually transmitted infections and sexual risk behavior among female sex workers in nine provinces in Indonesia, 2005. The Southeast Asian Journal of Tropical Medicine and Public Health, Vol.41, No.2, (Marchr 2010) pp. 463-473, ISSN 0125-1562
Tipples, G. & McClarty, G. (1993). The obligate intracellular bacterium Chlamydia trachomatis is auxotrophic for three of the four ribonucleoside triphosphates. Molecular Microbiology, Vol.25, No.1, (June 1993), pp. 1096-1194, ISSN 1365-2958
Twin, J.; Moore, E.; Garland, S.; Stevens, M.; Fairley C.K; Donovan, B.; Rawlinson, W.; Tabrizi, S. (2010). Chlamydia trachomatis Genotypes Among Men Who Have Sex With Men in Australia. Sexually Transmitted Diseases, (November 2010), ISSN 1537- 4521
Van Dyck, E.; Ieven, M.; Pattyn, S.; Van Damme, L.; Laga, M. (2001). Detection of Chlamydia trachomatis and Neisseria gonorrhoeae by enzyme immunoassay, culture, and three nucleic acid amplification tests. Journal of Clinical Microbiology, Vol.39, No.5, (May 2001) pp. 1751-1756, ISSN 1098-660X
Wang, S. & Grayston J. (1970). Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. American Journal of Ophthalmology, Vol.70, No.3, (September 1970) pp. 367-374, ISSN 1879-1891
Wang, Y.; Skilton, R.; Cutcliffe, L.; Andrews, E.; Clarke, I.; Marsh, P. (2011). Evaluation of a
high resolution genotyping method for Chlamydia trachomatis using routine clinical samples. Public Library of Science one, Vol.6, No.2, (February 2011) pp. e16971, ISSN 1932-6203
Watson E.; Templeton, A.; Russell, I.; Paavonen, J.; Mardh, P.; Stary, A.; Pederson, B. (2002).
The accuracy and efficacy of screening tests for Chlamydia trachomatis: a systematic review. Journal of Medical Microbiology, Vol.51, No.12, (December 2002) pp. 1021- 1031, ISSN 1473-5644
World Health Organization. Prevention and control of sexually transmitted infections: draft global strategy. [http://www.who.int]. WHO, 2006.
World Health Organization. Global prevalence and incidence of selected curable sexually transmissible disease: overview and estimates. Genoveva. WHO, 2001.
Yang, B.; Zheng, H.; Feng, Z.; Xue, Y.; Wu, X.; Huang, J.; Xue X.; Jiang H. (2010). The prevalence and distribution of Chlamydia trachomatis genotypes among sexually transmitted disease clinic patients in Guangzhou, China, 2005-2008. Japanese Journal of Infectious Diseases, Vol.63, No.5, (September 2010) pp. 342-345, ISSN 1344-6304
Yu, M.; Li, L.; Li, S.; Tang, L.; Tai, Y.; Chen, K. (2007). Molecular epidemiology of genital chlamydial infection among male patients attending an STD clinic in Taipei, Taiwan. Sexually transmitted diseases, Vol.34, No.8, (August 2007), pp. 570-573, ISSN 1537-4521
Yuan, Y.; Zhang, Y.; Watkins, N.; Caldwell, H. (1989). Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infection and Immunity, Vol.57, No.4, (April 1989) pp. 1040-1049, ISSN 1098-5522
Zimmerman, H.; Potterat, J.; Dukes, R.; Muth, J.; Zimmerman, H.; Fogle, J.; Pratts, C. (1990). Epidemiologic differences between chlamydia and gonorrhea. American Journal of Public Health, Vol.80, No.11, (November 1990) pp. 1338-1342, ISSN 1541-0048
Znazen, A.; Frikha-Gargouri, O.; Berrajah, L.; Bellalouna, S.; Hakim, H.; Gueddana, N.; Hammami, A. (2010). Sexually transmitted infections among female sex workers in Tunisia: high prevalence of Chlamydia trachomatis. Sexual Transmitted Infections, Vol.86, No.7, (December 2010) pp. 500-5005, ISSN 1472-3263



Comments
Post a Comment